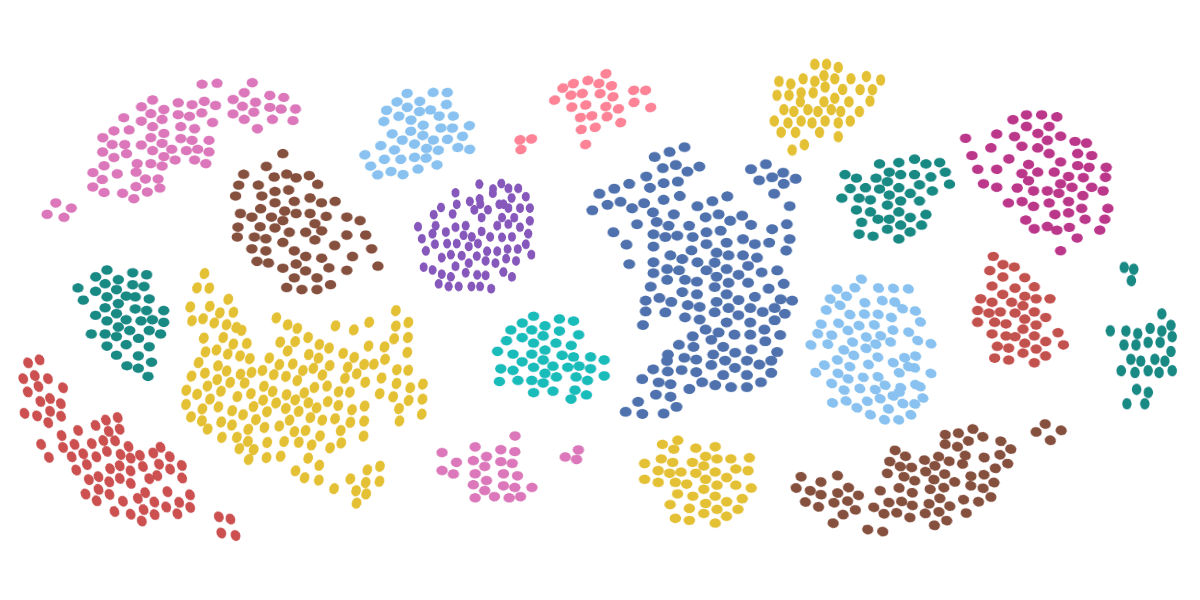
Flow Cytometry Testing: Determining an Individual Cell’s Immunophenotype
Lymphoma, leukemia, and myeloma are three types of major blood cell cancers. To determine whether a patient is suffering from one of these, a sample of blood is drawn and analyzed under one of two methods:
- Flow cytometry
- Immunocytochemistry
Both use immunophenotyping to differentiate blood cells and analyze the results to determine if there are cancer cells within the sample. In this article, the former method will be examined as it relates to determining an individual cell’s immunophenotype.
While cancer tests are not its sole purpose, it is one major application that shows why flow cytometry is a powerful tool in cell biology.
What is Immunophenotyping?
Immunophenotyping, in its most basic form, is the technique or method of studying protein expression of cells.
Within cancer testing and research specifically, immunophenotyping consists of using antibodies to label white blood cells and then running the fluorescently labeled cells through a flow cytometer. The FSC and SSC results (forward scatter; side scatter) can detect subtle differences in healthy and cancerous blood cells.
A flow cytometry test via a flow cytometry machine not only differentiates between the types of cancer cells, but also can quantify the cells to determine severity or aggressiveness of the cancer.
The Power of Flow Cytometry in Immunophenotyping
An important distinction about the basic principles of flow cytometry is its practicality.
When performing any real-world test (as opposed to theoretical biology), rarely will you work with a homogenous sample. Blood, saliva, bone marrow—these aren’t singular substances. Instead, they’re heterogeneous fluids that contain large quantities of different cells. Flow cytometry grants an automated way to sort, isolate, and quantify these differing cells.
In fact, flow cytometry is used as a tool to streamline the CRISPR process. To learn more about what is CRISPR technology, visit our article explaining the process.
In a paper, Standardizing immunophenotyping for the Human Immunology Project, Maecker writes:
The study of cells moving in suspension through an image plane — flow cytometry — is a potent tool for immunology research and immune response monitoring. Its main advantages are that it makes multiparameter measurements and that it does so on a single-cell basis. The result is that this technique can dissect the phenotypes and functions of cell type subsets in ways that are not possible using a bulk assay, such as Western blots, microarrays or enzyme-linked immunosorbent assays (ELISAs). Nowhere has this proven more useful than in a mixed suspension of immune cells, such as the blood. Newer instrumentation allows for the analysis of eight or more parameters at flow rates of thousands of cells per second; the resulting rich data sets are unparalleled for the knowledge of immune function that they have contributed.
This is high praise for a technique (and machine: the flow cytometer) that has been iteratively improved upon since 1953. Let’s break down what Maecker said to see exactly why it’s such an effective tool for immunophenotyping.
1) “Multiparameter Measurements…”
To begin, this test stands out as an exceptional method of understanding cell characteristics because of the multiparameter measurements that are possible. Immune system cells, including an abnormal cell, can be differentiated by looking at the cell surface of proteins. However, with the number of overlaps of these proteins, a method is needed to do multiple measurements simultaneously. To understand the basics behind this, the three main parameters are:
- Forward Scatter (FSC) – Forward scatter refers to the light that shines past the cell in question into the detector behind it. Depending on the refracted light, you can determine the cell’s relative size compared to the others in the sample.
- Side Scatter (SSC) – Additionally, the light that refracts off the cell at a 90-degree angle runs through multiple filters calibrated for different wavelengths and reflects into photomultiplying tubes for detection. This refracted light speaks to the complexity of the cell.
- Fluorescent Labeling – By placing fluorescently-labeled antigens into your cell population, you can determine which cell is which because the laser will excite the fluorochromes, thus emitting photons to be collected by the detectors.
Putting these parameters together allows for an incredible amount of application across different biological and medical fields.
2) “On a Single-Cell Basis…”
Imagine trying to study a cluster of microscopic cells by running them through a laser to measure their light refraction. Unless you’re isolating each cell individually, the practice is moot. There’s variability within individual cell populations, for example in blood…
- Monocytes are typically the largest normal cells found in the blood, averaging between 15 and 18 µm in diameter.
- Lymphocytes are white blood cells that average between 7 and 10 µm on the smaller end but can be as large as 14 to 20 µm in diameter.
Without proper detection and analysis methods, these values quickly derange any hope of flow cytometry lab tests being practical (that word, again, is important). Secondly, if there was no control over the measurement system, these monocytes and lymphocytes would be streaming through the laser simultaneously, not to mention the other neutrophils, eosinophils, red blood cells, and the cancerous cells joining in on the party. The light scattering in all directions would create a cohesive slab of chaos.
There’s only one solution, the cells have to pass the laser one at a time.
See the problem here?
Cells are on a microscopic level. There’s no manual way to line them up single file. Instead, properties of fluid dynamics are utilized to achieve this.
- Sheath Fluid – There is a saline-based solution running through the flow cytometer. It doesn’t refract the laser to any relevant measurable degree (the equivalent of noise in a system), however it does run at laminar flow.
- Laminar Flow – When a fluid is running at laminar flow, it means that the layers of fluid don’t mix. Instead, the fluid streamlines through the machine in parallel unidirectional layers. When a heterogeneous fluid is injected into the sheath fluid at laminar flow, the fluid doesn’t mix.
- Hydrodynamic Focusing – As the cytometer funnels toward the laser, the stream gets thinner and the sheath fluid in laminar flow forces the heterogeneous fluid into a single-file line. This is hydrodynamic focusing.
Depending on the pressure of the core stream, this will help to negate doublets (when two cells run through the laser simultaneously). The lower pressure systems are slower, though they tend to work more efficiently.
3) “Dissects the Phenotypes and Functions of Cell Subsets…”
The cells are passing through the laser one at a time. Their light refraction is being measured using forward scatter and side scatter, and the fluorescent labeled dyes help isolate certain cell populations. Now it’s time to determine the phenotypes and functions of the cell subsets. These properties can be ascertained using:
- Single parameter histograms – Sorting cells based on positive and negative excitation to measure a single parameter.
- Dual parameter histograms – Creating a four-quadrant graph of positive-positive, positive-negative, negative-positive, and negative-negative, these histograms can measure two parameters at once.
- Density plots – With large amounts of data, density plots are useful for differentiating types of cells before the analysis process.
4) “Mixed Suspension of Immune Cells…”
Performing a flow cytometry blood sample test, or frankly any flow cytometry lab test, will involve a heterogeneous solution. In the case of blood, you are going to have a mixed suspension of various immune cells that would otherwise not be able to stay separated.
5) “Analysis of Eight or More Parameters…”
Apart from being able to measure multiple parameters at once, Maecker claims that the newer instrumentation can analyze eight or more parameters. Question is, what kind of parameters are these (besides the ones mentioned above), and how are they used?
- Cell viability – When you introduce a pathogen into a cell population, one thing you want to measure is the cell viability after the fact. For this, you can use a DNA binding dye, which will enter the cell plasma after it has been compromised. Running this through the flow cytometer will allow you to differentiate dead cells, and whether they’re in the early or late stages of cell death.
- Drug response – Similar to cell viability, you can measure the drug response and how it affects certain cells. This doesn’t include solely whether the cells live or die, but rather if there is a variation in proliferation, protein-absorption, health and vitality of the cell, etc. It all comes down to finding the proper antigens to bind to the organelles you want to measure.
- Cell cycle – Considering that the content of the DNA varies depending on which of the four stages of the life cycle a cell is in, different antigens can be used as DNA binders. For this reason, cells can be sorted based on their cell cycle stage and then analyzed individually.
- Pathways of cell death – Flow cytometry can help understand the stages of cell death as well as cell life. Depending on which pathway of cell death you want to measure, different biochemical and morphological changes can be targeted.
-
- Apoptosis – This cell death is characterized by cell shrinkage before it disassembles or breaks apart.
-
- Oncosis – In an opposite manner, oncosis involves cell swelling until it ruptures.
6) “Flow Rates of Thousands of Cells Per Second…”
The word, again, to come back to is practical. Flow cytometers can perform all of the above functions and provide invaluable data across multiple fields at an incredible speed. Thousands of cells per second can flow through the laser, be recorded for analysis, and then be sorted for further testing.
This is where flow cytometry strides ahead of any other technique that is available for these types of measurements.
The Future of Flow Cytometry and Immunophenotyping
Of course, with the ever-advancing landscape of technology, certain flow cytometers will be more efficient at immunophenotyping than others. If you want a machine that will perform flow cytometry lab tests with incredible efficacy, there’s NanoCellect’s WOLF Cell Sorter.
Not only will you be able to determine a cell’s immunophenotype, but you can do so without worrying about killing a chunk of your sample cells in the process. The WOLF Cell Sorter was designed to be ten times more gentle on cells than the next leading gentle flow cytometer. This provides the opportunity to continue testing after your cells have been sorted.
At NanoCellect, we know happy cells.
Sources:
- AACC. Immunophenotyping. https://labtestsonline.org/tests/immunophenotyping
- NCBI. Standardizing immunophenotyping for the Human Immunology Project. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3409649/
- Excyte. 4 Fluidics Tips That Will Change Your Flow Data For The Better. https://expertcytometry.com/fluidics/
- News Medical Life Sciences. Flow Cytometry Measurable Parameters. https://www.news-medical.net/life-sciences/Flow-Cytometry-Measurable-Parameters.aspx
- Biocompare. Flow Cytometry Streamlines CRISPR Process. https://www.biocompare.com/Editorial-Articles/346273-The-CRISPR-Caveat/