Analyzing Flow Cytometry Results: Everything You Need to Know
Flow cytometers utilize properties of fluid dynamics to send cells one at a time through a laser. The optics and computer systems then track the photon emission from excited cells and analyze both the light that scatters past (forward scatter; FSC) and the light that scatters perpendicularly (side scatter; SSC). This allows researchers to identify multiple characteristics of a cell simultaneously.
The results, however, don’t provide exact descriptions or models of the cell. Instead they offer relative information (‘X’ cells are larger than ‘Y’ cells) and differentiating traits (‘G’ cells are more granular, more complex, than ‘H’ cells).
To understand how to interpret flow cytometry results through a flow cytometer machine, read ahead.
Flow Cytometry Results Interpretation | Seurat and the Cell Sorting Impressionist
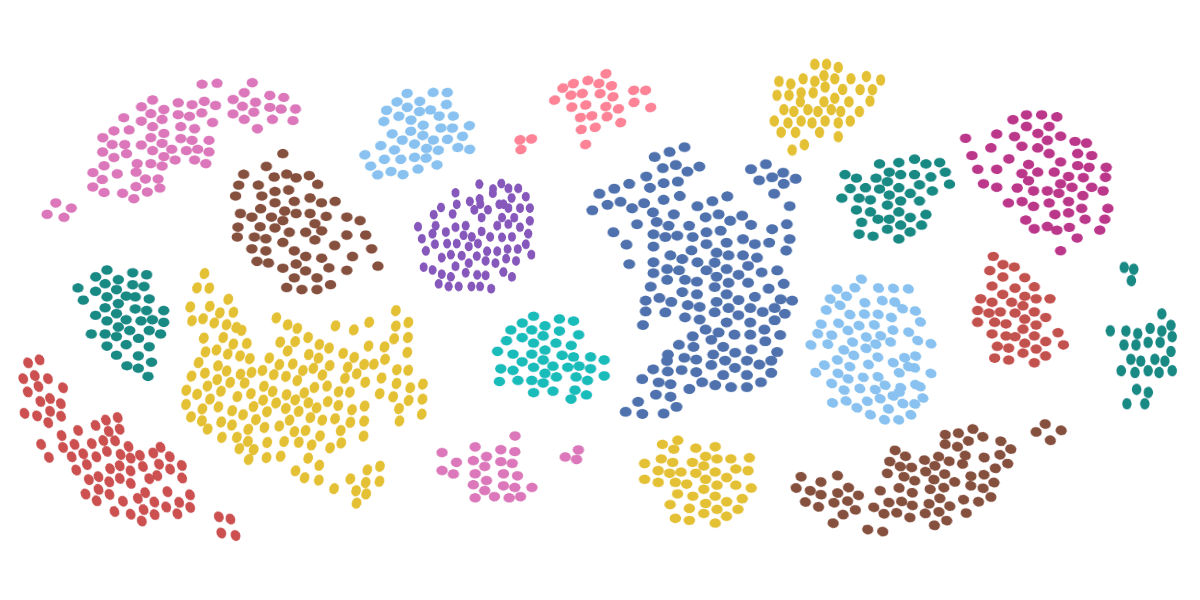
If you’ve never seen flow cytometry results before, your first guess might be that the ghost of Seurat lives inside your machine. A scattered buckshot of dots color the graph in a manner that looks closer to Sunday target practice than science.
To the trained eye, however, these seemingly randomized data points represent physical properties and internal characteristics of a cell.
Welcome to the scientific equivalent of impressionism…
Part 1 | Gathering the Data Correctly
As a student—a Scientifique-in-training—you’re always eager to jump in, solve the mystery at hand, patent your ingenious mind, and humbly accept your Nobel prize. Thank you, thank you, I have no idea what inspired me.
As a scientist—a hardened mind—you know gathering data, the less sexy side of science, is not just vital, it’s everything.
If you don’t gather proper data, analyzing the results will be fruitless. And with flow cytometry, there are many ways in which you can gather data improperly. Let’s discuss a few prerequisites before jumping into the interpretation of flow cytometry results.
Setting Up The Electronics
To properly set up your electronics system, the first step is to determine the parameters. You want to ensure the proper area is covered to see the full set of scattered light hitting the detectors, but you also don’t want to be so zoomed out that your collection of data combine into one point.
What might need tweaking?
- Photomultiplier tubes: changing the voltages
- Discriminator: changing the threshold
- Linear vs logarithmic data
- Setting gates or creating regions for data collection
Keeping Cells Alive
When measuring and sorting cells, you want to keep them alive. However, with the pressures of standard cell sorters and flow cytometers, it’s common to lose a chunk of your cell population with each test. To avoid this, the NanoCellect WOLF Cell Sorter uses less than 2 psi of pressure throughout the analysis.
To put this in perspective, this is thirty times gentler than the conventional flow cytometers commonly found in labs.
Part 2 | Gating the Data
Once you’re set to gather the data, the next step is to gate the data. Setting up the proper parameters within the electronics and the flow cytometer allows you to properly collect the data; gating ensures that what you’re looking at is helpful. It singles out the cells that you want to further analyze.
Some basic techniques when gating data include:
- Understanding the theory behind the cells in question beforehand. To create boundaries for your data, you’ll need to know the basic dimensions of the cell: size, complexity or granularity of the cells, what markers the cells express, etc.
- Use forward scatter and side scatter data to exclude dead cells and unwanted events. Three of the common data errors can be accounted for by dead cells, debris, and two cells moving through the laser at once (doublets). All of which can result in unexplainable events, thus, the reason to gate them out. Forward scatter estimates size of cells and, as such, can be used to ignore the data that would not correlate to the cells you wish to focus on.
- Gate for single or dual parameters. Creating a histogram to analyze the data (detailed further in Part 3) can be done with single parameters (such as positive and negative populations of fluorescent markers) or dual parameters can be analyzed on bivariate scatter plots.
Once the gates are in place and the data is collected, all that’s left is to look at the final results.
Part 3 | Analyzing the Data
Finally, the pièce de résistance, the stuff of laureates, piecing together the mysteries of your heterogeneous fluid. While there are an incredible number of specific techniques designed for different fluids, here, general techniques will be explained. Beginning with the underlying mathematical component, the method behind the madness, statistical analysis.
Statistical Analysis
To properly ascertain information from large data sets, statistics and statistical analysis is a must. Theoretical models provide tidy equations with predictable results. Real-world data, on the other hand, would never prove a theoretical model’s efficacy without generalizations.
And that’s exactly what statistical analysis provides.
Liken its effect to the flip of a coin. It’s painfully obvious the chances of ‘heads’ or ‘tails’ are 50-50. However, after flipping the coin ten times, you may think that the theoretical model fails. Only after flipping the coin a thousand times, or a hundred thousand times, does the distribution model start to reflect the 50-50 theoretical model.
Same goes for analyzing cells. The resulting data set will be a distribution of sizes and granularity. Gating the data properly and knowing the approximate cell size beforehand will make the clusters of data stand out.
For example, looking at a forward scatter dot plot of white blood cells: It’s easy to identify that the smaller data set is likely the 7 to 10 µm Lymphocytes, whereas the larger data set is likely the 15 to 25 µm Monocytes.
A histogram will provide distribution curves of the data. Using two basic principles of statistical analysis—intensity and spread—will help explain their properties.
- Intensity – The intensity on a density plot is quite literally, where the color and collection of data is most intense. Mathematically speaking, the intensity is closer to the mean or median of the peak. It locates the true center of the distribution. With enough data points, the mean intensity will align with correct theoretical models.
- Spread – The spread, on the other hand, is what represents the value differences of a cell. While a cell may be, on average, 15 µm, it’s helpful to know whether it’s equally common to find a 13 µm, 18 µm, or 15 µm cell, or whether most of the cells are going to be a certain size. This comes from understanding the standard deviation, or with flow cytometry specifically, the coefficient of variation—the standard deviation divided by the mean channel number.
Forward Scatter vs. Side Scatter
As mentioned previously, forward scatter (FSC) and side scatter (SSC) can help to differentiate unwanted events in the data, but this doesn’t speak to the extent of their usefulness. In their most basic form, the two scatter plots can be generally defined as:
- Forward scatter – The light that scatters past the cell will denote the diameter and volume of the cell.
- Side scatter – The light scatter will inform about the granularity or complexity of the nucleic structure within the cell.
But again, this is only the beginning. Plotting the forward scatter height against the width will allow you to separate doublets from the data pool. If two cells simultaneously pass through the laser, the height might be consistent with the other cells, but the area would be twice as large. Thus, a doublet.
Histograms | Analyzing Single Parameters
Single parameter histograms are useful for identifying any fluorescent marker expression within a population. It separates the cells into a positive and negative category, which could be useful in determining any number of characteristics of the heterogeneous fluid.
Density Plots | Analyzing Dual Parameters
To further analyze your cell population, density plots—or bivariate scatter plots—allow for combinations of dyes to be analyzed simultaneously. This is particularly effective in heterogeneous fluid, like blood or saliva, where the number of different cell populations within the sample is high.
From Population to Presentation | NanoCellect
Once you’ve analyzed the data, it’s time to form your conclusion. Whether you’re analyzing a sample looking for cancer cells, or separating stem cells in a laboratory, flow cytometry is the most effective method for counting and sorting heterogeneous fluids but its efficacy depends heavily on the machinery used.
That’s why more labs are turning to NanoCellect for their cutting-edge flow cytometry technology. Not only is their WOLF Cell Sorter gentler than any other flow cytometer on the market, it is also:
- Contaminant and biohazard free – Because it uses disposable cartridges, you don’t have to worry about any sample getting cross contaminated.
- Easy and intuitive – The machine itself is straightforward, and the software can be understood by beginners and professionals alike.
- Conveniently sized – Coming in under two cubic feet, the WOLF Cell Sorter can fit into any laboratory, in any space.
Go from population to presentation with ease, and redefine the way you think about flow cytometry, with NanoCellect.
If you’re interested to know about WOLF Cell Sorter applications such as single-cell DNA sequencing, antibody discovery, gene editing and cell line development. Contact us today!
Sources:
- Anatomy Atlases. Section 4: Blood. https://www.anatomyatlases.org/MicroscopicAnatomy/Section04/Section04.shtml
- Bitesize Bio. Data Spread and How to Measure It: the Coefficient of Variation (CV). https://bitesizebio.com/22776/data-spread-and-how-to-measure-it-the-coefficient-of-variation-cv/
- Bio-Rad. Flow Cytometry Data Analysis. https://www.bio-rad-antibodies.com/flow-cytometry-gating-strategies.html
- Flow Cytometry: A Basic Introduction. Chapter 4: Data Analysis. https://flowbook.denovosoftware.com/chapter-4-data-analysis