Using CRISPR Technology to Engineer Genetically Modified Cell Lines
CRISPR first tapped into the mainstream consciousness when Chinese biophysicist, He Jiankui, used the genetic engineering technique to “edit” two twin girls for HIV resistance. The ensuing headlines raged—the ethics and morality of what Jiankui did were fiercely debated.
On one hand, the emotional outrage was completely justified. With ethical and scientific norms put to the wayside, Jiankui took human life into his own hands. On the other hand, some claim he demonstrated exactly where CRISPR technology will inevitably lead humanity—and tackling the philosophy of science was shown to be dearly needed.
Today, in most labs across the globe, CRISPR is not a hand that writes humanity’s narrative. Instead, it’s a simple technique of genetically modifying human cell lines for cancer cell research, immunotherapy, cell biology, further analysis after using the flow cytometry machine, and more.
To unpack this technology and learn more about its uses, read ahead.
What is CRISPR Technology?
To begin, let’s break down CRISPR at its molecular level. And by that, let’s see what’s behind the acronym:
- C – Clustered
- R – Regularly
- I – Interspaced
- S – Short
- P – Palindromic
- R – Repeats
These clustered regularly interspaced short palindromic repeats are found in bacterial DNA, and they are copies of viruses (or pieces of viruses). The bacteria is able to use the repeats to identify harmful viruses and then attack them. The enzyme that physically tears apart the viral DNA strand is known as CRISPR-associated 9 (or Cas9).
After observing the functionality of these bacterial enzymes, scientists then discovered how to replicate it for their own use. From there, they discovered how to remove, add, or (perhaps most importantly) alter existing DNA, thus editing a genome.
CRISPR-Cas9 is now the most accurate, cheapest, and fastest-working genome-editing method available today. It is what people refer to as “CRISPR.”
CRISPR | How it Works
DNA is made up of nucleotides bonded together in pairs. To take you back to high school biology: adenine pairs with thymine (A–T) and guanine pairs with cytosine (G–C). These simple chained nucleotides form a long double-helix chain that is unique to every organism. These double-helix chains build up chromosomes, nuclei, and determine everything from the color of your eyes to your maximum potential height.
CRISPR (again, shorthand for CRISPR-Cas9), utilizes the Cas9 enzyme, a naturally produced protein in cell types built for DNA splicing, to “unzip” these chained nucleotides at a specific spot and then replace the nucleotide chain with the one attached. The location is based on pre-programmed information in the enzyme—essentially it floats around inside the nucleus until it finds the correct spot, then gets to work.
How CRISPR Affects Cell Lines
Cell lines are cell cultures that will proliferate forever given the right conditions of space and medium. For example, CHO cells (Chinese hamster ovary cells) are widely used in practices from transfection to pharmaceutical drug creation to recombinant protein production. Despite the fact that these CHO cells are in almost every major laboratory across the globe, they are all from the same mammal—the same female Chinese hamster.
CRISPR is able to edit the cell culture, changing one piece of the genetic code, and then allow the morphological and physical characteristic changes to manifest. Thus, this makes CRISPR technology incredibly useful for genome editing.
Who Uses CRISPR
It’s not just geneticists and cell biologists that are vying for a spot at the CRISPR table; in fact, many industries utilize this technology to create genetically modified cell lines.
- Agricultural industry – Scientists working for agriculture corporations are trying to identify ways to use CRISPR to genetically modify crops to do everything from:
- Create fruits that don’t bruise or change color
- Enhance crops to include natural pesticide, herbicide, and fungicide qualities
- Strengthen crops to weather various storms (including the slow changes of global warming)
- Grow larger foods with more nutrients packed in
- Creating larger livestock to produce more meat per animal
- Pharmaceutical industry – CRISPR now plays a major part in the drug research process. On one end of the equation, the gene editing tool can help modify protein-building structures that allow for concentrated areas of study.
- Medical industry – Apart from the drug creation process, there’s also the case of genetic diseases that current physicians don’t have a way of treating aside from managing the symptoms.
- Alzheimer’s disease can be passed on from parent to child genetically, as stated by the NIH’s understanding:
“Some cases of early-onset Alzheimer disease are caused by gene expression mutations that can be passed from parent to child. This results in what is known as early-onset familial Alzheimer disease (FAD). Researchers have found that this form of the disorder can result from mutations in the APP, PSEN1, or PSEN2 genes. When any of these genes is altered, large amounts of a toxic protein fragment called amyloid beta peptide are produced in the brain.”
If the genes can be isolated within the genetic code itself, it’s possible that CRISPR will be able to remove or alter these genes to stop the production of amyloid beta peptide.
CRISPR also plays a role in flow cytometry. To learn more, visit our articles discussing the basics principles of flow cytometry and a flow cytometry test.
This might seem like too much power to be wielded by individual groups—and perhaps it is—however, gene editing as a whole is not brand new. There were gene editing tools long before CRISPR came along.
CRISPR Genome Editing | A Background Guide to How We Got Here
CRISPR is just one tool in a whole group of genome-editing technologies. Each tool in this microscopic toolbox has the ability to edit (add, remove, alter) a portion of a genome.
Gene editing as a field of study is not new. According to a report done by Dana Carroll on Gene Editing: Past, Present, and Future:
“In the mid-twentieth century, Muller and Auerbach demonstrated that the rate of mutagenesis could be enhanced with radiation or chemical treatment. Later methods relied on transposon insertions that could be induced in some organisms; but these procedures, like radiation and chemical mutagenesis, produced changes at random sites in the genome. The first targeted genomic changes were produced in yeast and in mice in the 1970s and 1980s.”
Carroll goes on to mention that the early gene targeting process done on mouse cells was precise, yet lacked in efficiency. And due to low embryonic stem cell numbers of in mammals (outside of mice), gene targeting and editing took longer to roll over to other species.
Now, the lack of resources isn’t the problem—in fact, quite the opposite. Now pushing forward too fast, too soon is more of a contentious issue.
Since then, the last 40–50 years has been filled with incredible advancements, including:
- Zinc Finger Nucleases (ZFN)
- Transcription Activator-Like Effector Nucleases (TALEN)
And of course, CRISPR.
ZFN vs CRISPR vs TALEN | What’s the difference
What marks the difference between these three besides a differing track record of about twenty years in practice? In an interview with Charles Gersbach, Ph.D. and professor of biomedical engineering at Duke, who’s worked with both ZFN, TALEN,and CRISPR, he states:
“Fundamentally, all these technologies do the same things. They cut DNA, and once you get past the point of cutting the DNA, all of the rest of the aspects of genome editing are the same.”
In some ways, Gersbach’s oversimplification is accurate. All three techniques, being gene editing tools, are methods of adding, removing, and replacing genetic code. But is there any difference at all?
- ZFN – This genetic modification technique was the first to enter the scene. These zinc finger moieties can recognize 3-6 DNA triplets, which is typically enough to find any precise genetic code.
- TALEN – Similar to ZNFs, TALENs utilize DNA binders to find the right sequence. However, TALENs recognize single nucleotides, not DNA triplets.
- CRISPR – The latest exciting gene editor doesn’t seek out DNA sequences. Instead, it uses guide RNA formulations, much like bacteria would, to cut out and replace a DNA sequence.
While CRISPR seems to be the hip new gene editing tool that puts the others to shame, that might not actually be the case. The future of CRISPR is still yet to be written into the genetic code.
Combining CRISPR With Flow Cytometry
One thing that’s certain is that CRISPR is incredibly flexible, efficient, and targeted at finding mutations and reordering DNA. To that end, they will continue to be increasingly common in cell line technology.
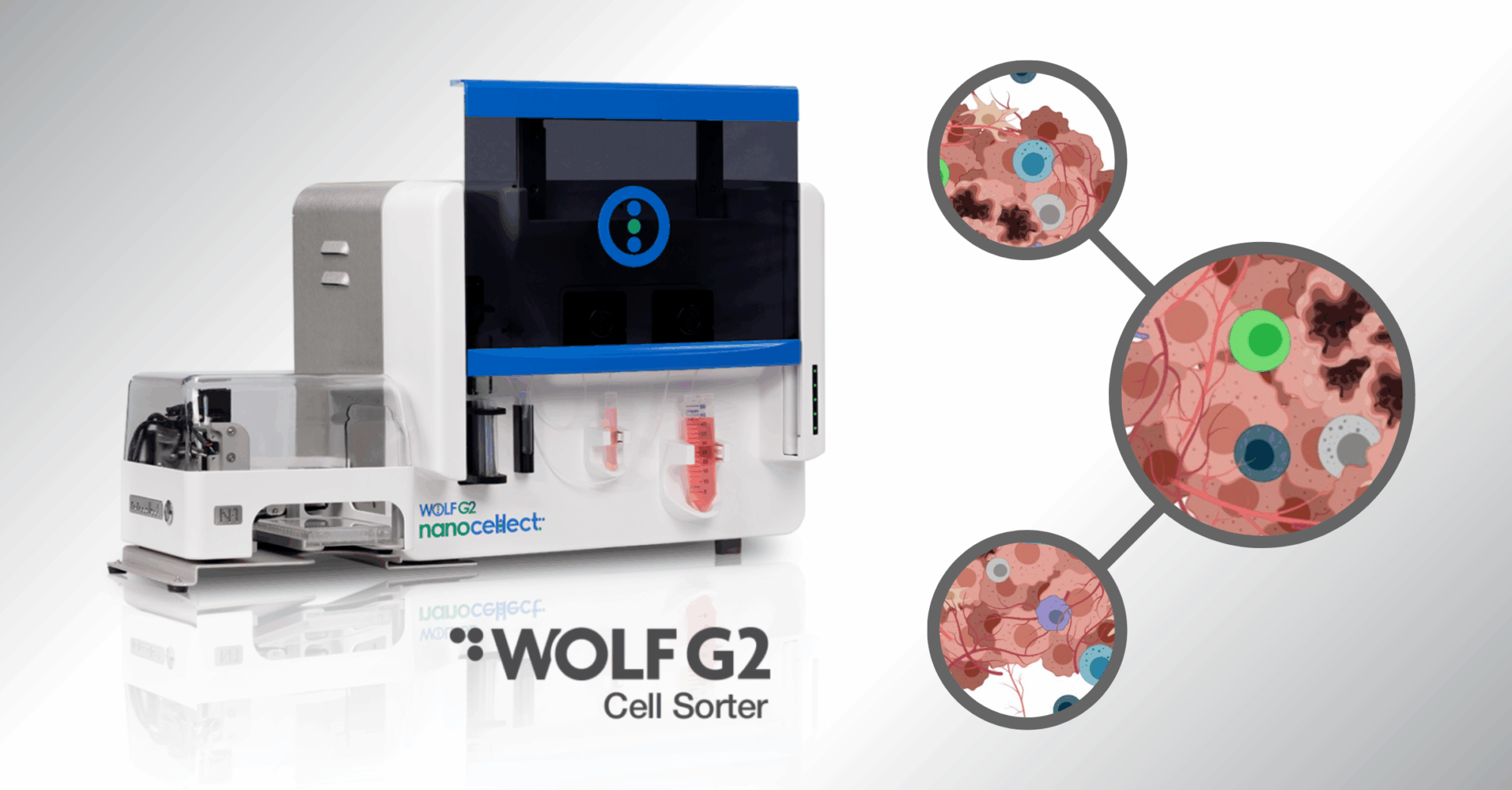
Similarly, new-age flow cytometer machines have been making headway in cell line development technology. The reason being is that it’s ideal to use flow cytometers for fluorescence activated cell sorting (FACS) to isolate cells once they have been edited. Cells can be sorted based on temporary fluorescent proteins that are integrated during the gene editing. CRISPR technology, though very efficient, does not edit every cell in a population which results in a mixed population. Without FACS, it is difficult to isolate a pure population to study and can lead to erroneous conclusions.
However, many cell types used for CRISPR gene editing are sensitive cells, like induce pluripotent stem cells (iPSCs), which die easily from the stress caused by traditional FACS machines. With the NanoCellect WOLF® G2 Cell Sorter, cells undergo pressures 30 times gentler than the industry standard. This keeps the population healthy and viable after sorting, which is crucial for downstream research.
To that end, new flow cytometry technology, like NanoCellect’s WOLF G2 Cell Sorter, has been creating waves alongside CRISPR and other cell line technology. Combining these two technologies make the gene editing tool even more potent in laboratories.
The Future of CRISPR
Using CRISPR to genetically modify a human embryo is still in the far-distant future, at least to any meaningful degree. In conclusion to the story of Jiankui and the two edited twin girls—the World Health Organization made headway with countries across the globe to halt gene-edited fetuses. China issued a mandate that anyone who did continue to genetically alter fetal DNA would face the legal ramifications of any adverse side effects.
While again, as seen above, this is not the only use of CRISPR (nor is it even the primary use)—this technology does have the potential to be the forefront philosophical debate of our time.
Until then, however, CRISPR with flow cytometry will remain a positive force in cell line technology.
Sources:
- MIT Tech Review. China’s CRISPR babies: Read exclusive excerpts from the unseen original research. https://www.technologyreview.com/s/614764/chinas-crispr-babies-read-exclusive-excerpts-he-jiankui-paper/
- Science Direct. Cell Lines. https://www.sciencedirect.com/topics/neuroscience/cell-lines
- NIH. Alzheimer disease. https://ghr.nlm.nih.gov/condition/alzheimer-disease#genes
- GEN. Are Zinc Finger Nucleases Making a Comeback? https://www.genengnews.com/insights/are-zinc-finger-nucleases-making-a-comeback/
- Wired. The World Health Organization Says No More Gene-Edited Babies. https://www.wired.com/story/the-world-health-organization-says-no-more-gene-edited-babies/