Isolate Antibody-Secreting CHO Cells for Single-Cell Cloning with the WOLF
Introduction
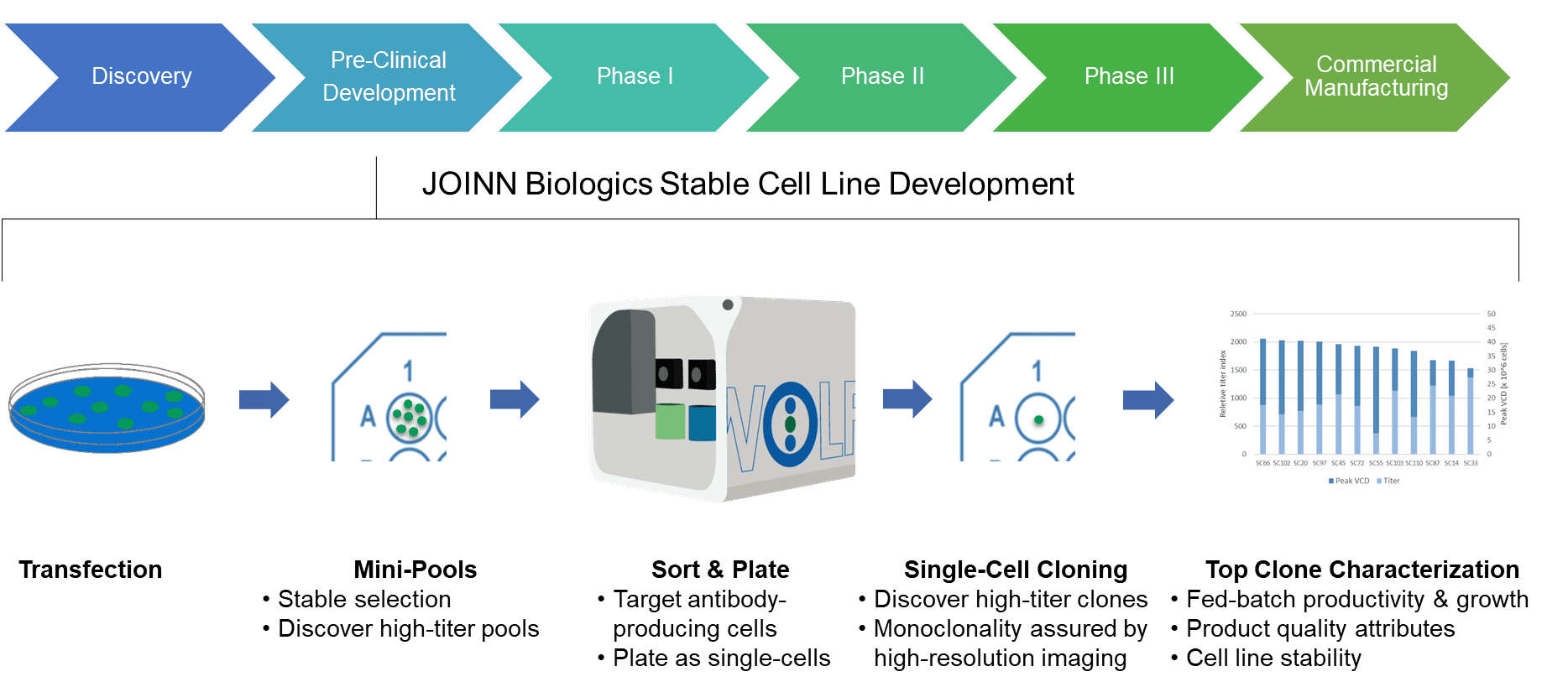
The therapeutic antibody discovery and development process can last over 10 years before reaching the commercial manufacturing stage. Therefore, it is critical to make every element of this process efficient. Transitioning from the discovery phase to pre-clinical characterization and manufacturing requires the development of a stable, high-producing monoclonal cell line. Isolating a single, antibody-producing clone can be time-consuming with traditional techniques such as limiting dilution, which can leave 41%-77% of wells empty and only 25%-43% of remaining wells yielding on-target single-cell colonies1. Cell printers can improve upon the low dispensing efficiency however, they still lack the ability to select for live, protein-producing target cells.
Fluorescence-activated cell sorters, such as the WOLF®, allow for the selection of single cells based on scatter and fluorescent tags that indicate protein or specific antibody secretion and viability. The N1 Single Cell Dispenser can then deposit single cells into 96- or 384-well plates with higher than 90% efficiency2. The low pressure of the system (< 2 psi) ensures gentle sorting and does not disturb the secretory activity of the cells. With a small, 2-cubic-foot form factor, the WOLF can easily fit into a biosafety cabinet to provide a sterile workspace. The disposable cartridge and fluidics are also sterile and ensure a workflow completely free of cell contamination.
This sterile workflow and the ability to select for high-producing cells are what make this a powerful tool to improve the efficiency of stable cell line generation in CDMOs (Contract Development and Manufacturing Organizations), like JOINN Biologics. JOINN Biologics provides a complete range of services, from early custom development to clinical manufacturing. Their JOINN Laboratories group focuses on the early development phase of biological therapeutics, including manufacturing cell line development. Their commitment to state-of-the-art technology platforms, coupled with CHO-based host cell systems, includes utilizing the WOLF to sort cells that are actively producing the recombinant antibody-of-interest, as demonstrated below.
Method
To simulate varying levels of transfection efficiency and evaluate the WOLF’s accuracy in detecting antibody-secreting cells, varying amounts of stably transfected CHO cells were mixed with wild-type. The transfected cells, which produce a recombinant human IgG antibody, were titrated at total concentrations of 100%, 50%, 20%, and 10%. All centrifugations, incubations, and media steps were kept at 4oC to minimize changes in secretory activity. Cells were centrifuged at 350 x g for 5 minutes, washed once, and resuspended at 1×105 cells/mL in media. Cells were incubated in the dark with PE-anti-human IgG for 20 minutes and washed twice with media. SytoxGreen was added at 2 drops/mL and incubated in the dark for 15 minutes.
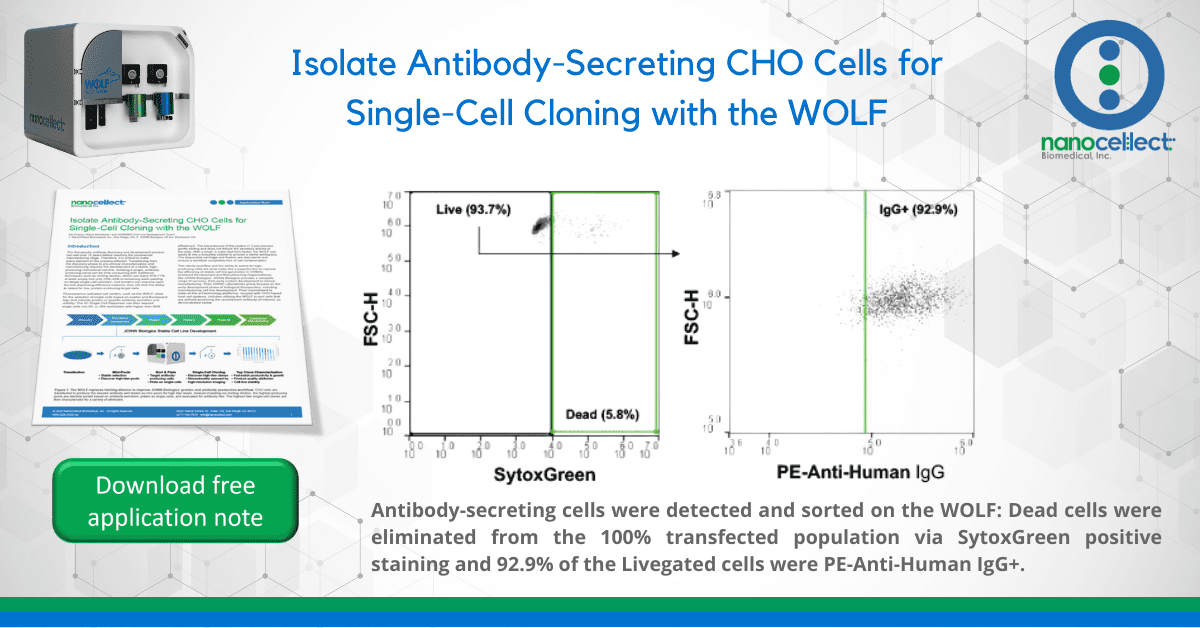
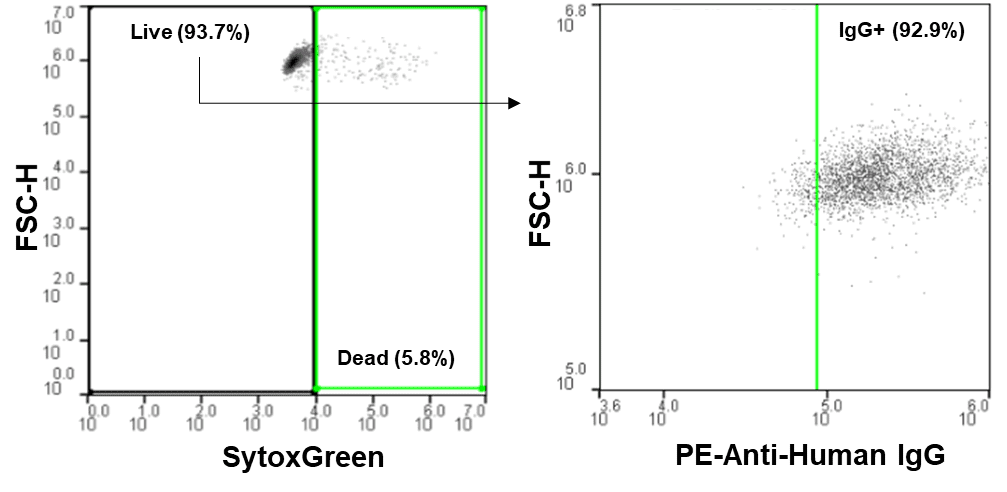
Cells negative for SytoxGreen and positive for PE-anti-human IgG were sorted at 4°C, using ice-cold media as sheath (Figure 2), and dispensed into 96-well plates with room temperature media. One plate was sorted and dispensed for each concentration and analyzed for outgrowth with the CloneSelect™ Imager. Wells with outgrowth were analyzed for Protein A binding with the Octet® Red96e.
Results
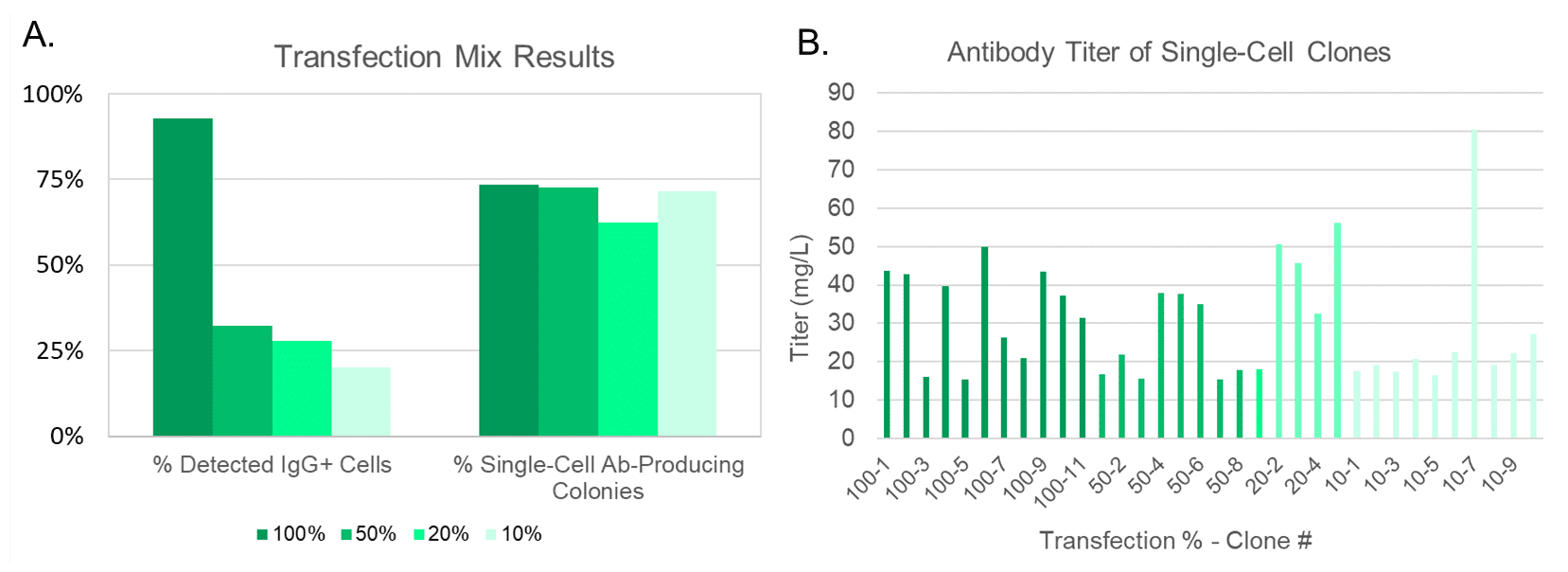
The WOLF was able to detect IgG+ cells for each concentration mix (Figure 3A, left) and yielded similar levels of single-cell antibody-producing colonies, despite the varying initial concentrations. On average, 70% of the cell outgrowth on each plate was from a single antibody-producing cell (Figure 3A, right). This on-target outgrowth is significantly higher than the average 43% (2.6% CV) seen in previous studies for a 0.5 cell/well limiting dilution with a very robust CHO cell line. The CV for outgrowth of single-cell producing clones was 7.2% across all four plates and the Octet Red96e confirmed titers of 15 to 80mg/L for each of the single-cell clones (Figure 3B).
Conclusion
The WOLF accurately detects and sorts antibody-secreting cells at target populations as low at 10% with maximum monoclonal outgrowth and titer levels. This methodology can expedite development of high-producing, clonal stable cell lines from novel transfections in the antibody production process. The WOLF’s fluorescence-activated cell sorting capabilities, gentle pressure, and sterile fluidics make it a powerful tool to accelerate therapeutic antibody workflows.
References
- Krasny, A. & Morachis, J. M. (2019). WOLF Exceeds Limiting Dilution Cell Cloning Efficiency. Retrieved from https://nanocellect.com/scientific-content/
- Krasny, A., Chen, H., Daniells, W., Bozek, E., Leary, B., Alaynick, W., & Morachis, J. M. (2019). Single-Cell Cloning for Cell Line Development. Retrieved from https://nanocellect.com/scientific-content/
APN -009


