WOLF Cell Sorter Purifies Neutrophils with High Viability
Introduction
Neutrophils play a critical role in chronic inflammation and have been associated with antimicrobial functions in acute infections. The half-life of circulating neutrophils is estimated at between 13-191 hours, however, if activated, they may be cleared even faster. The shear forces and pressure of traditional FACS can be harsh on short-lived, sensitive cells like these and, as a result, the use of magnetic beads and centrifugation gradients to isolate neutrophils is sometimes preferred.
However, these methods lack the ability to select for viability or multiple markers needed for more specific gating. For example, selecting for common neutrophil markers CD16 and CD66b will include some low-expressing offtarget cells, like monocytes. Therefore, complementary negative selection for CD14 and another neutrophilnegative marker, like CD49d, is advantageous. NanoCellect’s WOLF Cell Sorter allows for this multi-parameter detection and sorting like traditional FACS. However, the WOLF’s microfluidic sorting mechanism uses far lower
pressure (<2 psi vs 10-70 psi) that results in gentler sorting and healthier cells post-sort. Here, the ability of this easy-to-use instrument to sort neutrophils with high purity and minimal cell death was investigated.
Method
Sorting
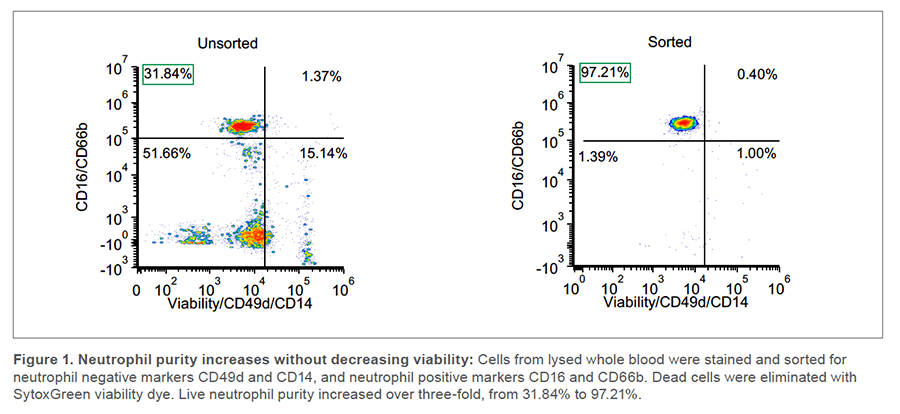
Whole blood was incubated with 1x RBC Lysis Buffer at 1:20 in the dark for 15 min at room temp (RT). After centrifugation at 350 x g for 5 min, the supernatant was removed, and cells were resuspended in HBSS/10% FBS. 100uL of cells were incubated 10 min with True-Stain Fc and Monocyte Blockers to prevent non-specific binding. FL1 was used as a dump channel with AF488-labeled CD49d and CD14 (R&D systems, #FAB1354G and Biolegend, #301811) to eliminate non-neutrophils. Positive neutrophil selection markers, PE-labeled CD16 and CD66b (Biolegend, #302008 and #305106), were combined for detection in FL2. After the antibodies were added, they were incubated for 20 min at 4oC. Cells were then washed, concentration was adjusted to 300 cells/uL, and SytoxGreen was added and incubated for 15 min at RT to stain dead cells. Cells were sorted at RT on the FL1neg/FL2high (live neutrophil) population (Figure 1).
Post-Sort Analysis
After sorting, the cells were centrifuged at 350 x g for 5 min and concentrated to 100uL. Fc and Monocyte Blockers were added again and incubated before adding AF488 CD49d/CD14 and PE CD16/CD66b. Cells were washed, incubated with SytoxGreen, and analyzed on the Accuri C6 to assess purity and viability.
Results
Purity & Viability
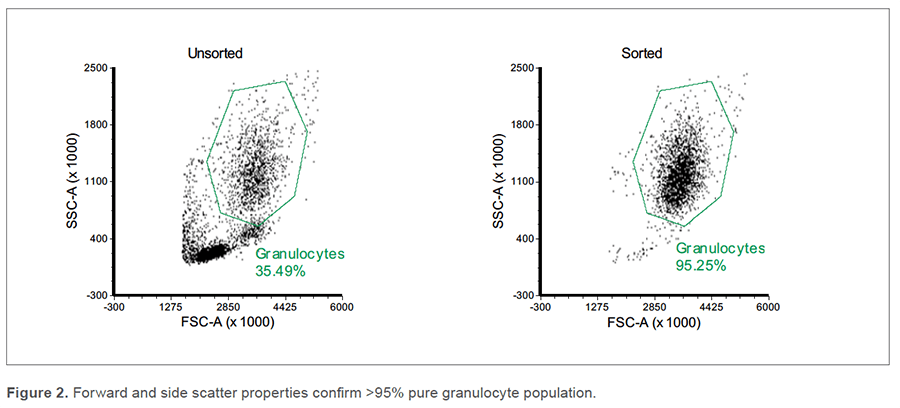
Post-sort analysis revealed that the purity of the live neutrophil population increased from 31.84% to 97.21% (Figure 1). Only 1.40% of cells remained FL1pos (AF488 CD49d/CD14/SytoxGreen) which indicates that, in addition to reducing non-neutrophils, sorting did not cause a significant increase in dead cells. The purity was also confirmed via scatter properties, with an increase from 35.49% to 95.25% in the granulocyte gate (Figure 2).
Conclusion
In summary, the WOLF Cell sorter can enrich for neutrophils with high purity and viability, making it an effective method for neutrophil separation. These results indicate that this novel microfluidic cell sorting technology can be used to study emerging neutrophil functions, mechanisms of action, and novel cell-based therapeutic
approaches.
References
-
Lahoz-Beneytez, Julio, et al. “Human Neutrophil Kinetics: Modeling of Stable Isotope Labeling Data Supports Short Blood Neutrophil Half-Lives.” Blood, vol. 127, no. 26, 2016, pp. 3431–3438., doi:10.1182/blood-2016-03-700336.
APN-021