The CS1 Chiller-Stirrer: A Valuable Tool for Improved Cell Viability with Temperature Sensitive Cells
Introduction
The WOLF G2 Cell Sorter is a low pressure, microfluidic based cell sorter aimed to maintain the viability and functionality of cells post-sort. However, some cell lines may require additional conditions to maintain their viability and avoid a stress response, such as cold temperature to slow the rate of metabolic processes during cell sorting.1 To better serve the needs of our customers and their specific cell types, NanoCellect has launched the CS1 Chiller-Stirrer Accessory.
The CS1 is an accessory module that attaches to either the WOLF or WOLF G2 cell sorters and serves two main functions: chilling and stirring. The CS1 can chill both the sheath fluid and the input cell sample, maintaining a temperature as cold as 4ºC (Figure 1). Additionally, in conjunction with a magnetic stir bar, the CS1 can provide gentle agitation to the sample fluid, thus preventing cellular aggregation and sample settling. With chilling and stirring functions that can be used either together or independently, the user has a variety of options and settings to choose from, providing flexibility for the needs of different cell types.
Here, we describe validation of the stirring and temperature settings of the CS1 and the benefits of using the CS1 when sorting Chinese Hamster Ovary (CHO) cells.
Methods
Sample Preparation
Suspension CHO-K1 cells (ATCC CCL-61) were expanded and maintained in Ham’s F12K media (Gibco 21127022) supplemented with FBS (Genesee Scientific 25-514H) and Antibiotic-Antimycotic (Gibco 15240096). Cells were prepared for sorting on the WOLF G2 Cell Sorter by resuspension in 1X PBS (Genesee Scientific 25-507), 1% BSA (Thermo Fisher 37525), 5 mM EDTA (Invitrogen 15575020), 12.5 mM HEPES (Gibco 1560080). The sample was filtered through a 35 µm filter cap prior to sorting (Genesee Scientific 28-155).
Validation of Temperature Settings
A temperature probe was placed into the sheath tube containing 1X PBS (Genesee Scientific 25-507) on the CS1 sample holder (Figure 1). The chiller was turned on and set to 5°C. After 30 minutes, the temperature was measured to ensure that the chiller reached the set temperature. The chiller was then tested for temperature stability. The temperature probe was left inside the sheath buffer and a temperature reading was collected after 12 hours to determine if the chiller remained at the same temperature.
Validation of Stirring Function
CHO-K1 cells were diluted at a final concentration of 2.0 x 106 cells/mL in PBS/1% BSA (Thermo Scientific 37525). The cells were then stirred at 200 rpm at room temperature using the stirrer function of the CS1 module. The stirrer function was turned on and the sample was analyzed on the WOLF G2 every 15 minutes for one hour to measure if the cell concentration remained consistent while stirring.
Sorting on WOLF® G2
CHO-K1 cells were analyzed on the WOLF G2 Cell Sorter and were gated for live singlet populations. Live singlet cells were bulk sorted for one hour with the CS1 chiller set to 4ºC without stirring or with interval stirring set to stir the cells for 5 seconds at 200 rpm every 10 minutes. Cell viability was measured by staining with Propidium Iodide Ready Flow Reagent (Invitrogen R37169) and measuring the percentage of live cells post-sort using the Novocyte Flow Cytometer (Agilent Technologies) and the Countess II (Thermo Fisher Scientific). As a control, one aliquot of CHO cells was left at room temperature for the duration of the one-hour sort.

Figure 1. WOLF G2 Unit with CS1 Attachment: The settings for the CS1 integrate into the existing WOLFViewer Software and allow for temperature and interval stirring settings to be adjusted easily by the user. Sample holders sit behind plastic casing (in blue and green) to help maintain the chilled temperature of the unit.
Results
Validation of Temperature and Stirring Settings
To demonstrate that the CS1 Chiller-Stirrer can hold a specific temperature for 12 hours, the temperature of the CS1 was set to 5°C for 12 hours with both sheath and sample buffer loaded on the instrument. The temperature was shown to be consistent for both sheath and sample tubes over the course of 12 hours (Figure 2).
While using the CS1 to stir samples, sample concentration remained steady for the first 45 minutes. In comparison, the sample concentration plummeted by 75% within the first 15-minutes when the sample was not stirred (Figure 3).
The Effect of Chilling and Stirring on Sample Viability
In comparison to the cells left at room temperature during the one-hour sort, cells on the CS1 with the chilling temperature set to 4ºC maintained high cell viability (Figure 4). While the CHO-K1 cells on the CS1 had an average drop in viability of only 1.5%, CHO-K1 cells at room temperature experienced a 24% drop in viability. When implementing the interval stirring function at 200 rpm for 5 seconds every 10 minutes over the course of the one-hour sort, post-sort viability remained above 90% (Figure 4). The average drop in post-sort viability after one hour of interval stirring while sorting was only 6.6%. While this drop in viability is greater than what is observed without stirring, it is still considerably better than the viability of the cells at room temperature.
However, it is for this reason that we recommend using the stirring function to maintain the suspension of only robust cells and using interval stirring rather than constant stirring throughout the duration of the sort.
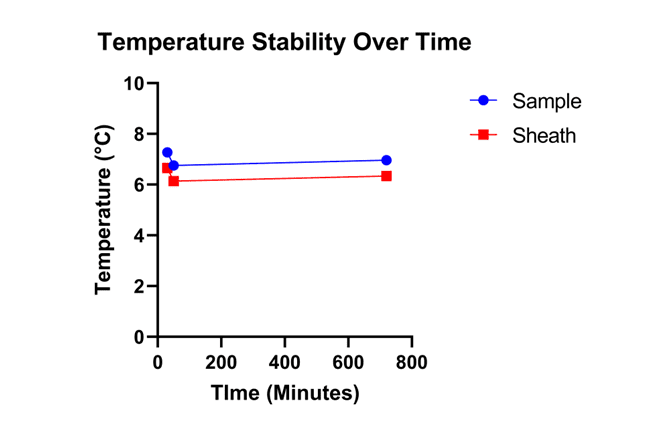
Figure 2. Temperature Stability of the Sample and Sheath Buffer: The red line shows the change in the temperature of the sheath buffer over the course of twelve hours. The blue line shows the change in temperature of sample buffer over the course of twelve hours.
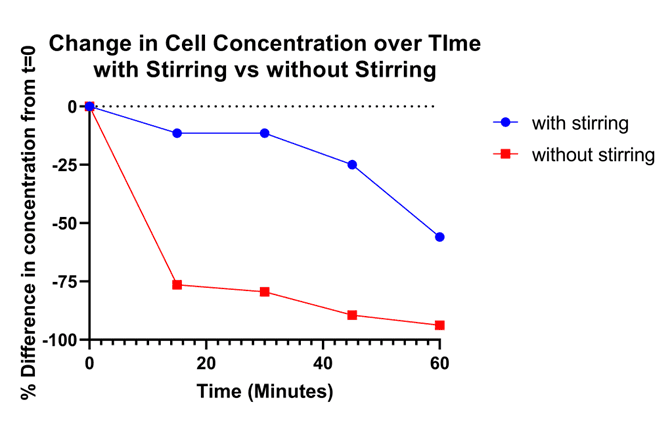
Figure 3. Sample Concentration Stability: The line in blue shows the percent decrease in measured concentration of cells over time with stirring. The red line shows the percent decrease in cell concentration over time without stirring.
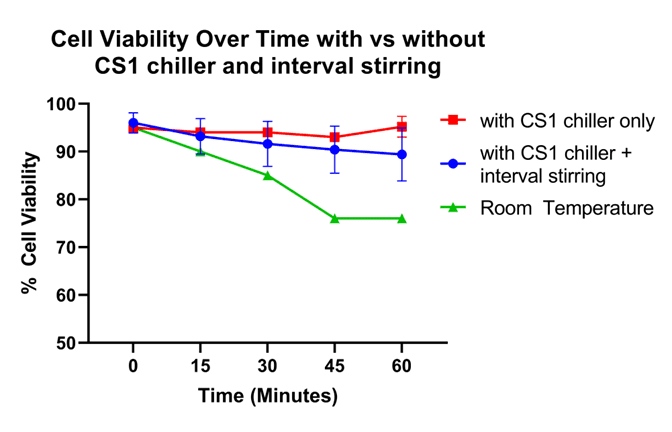
Figure 4. Cell Viability over time with and without chilling and stirring. The cell viability of CHO cells was measured over time: The red line shows the change in cell viability over the course of one hour with the CS1 set to 4°C and no stirring. The blue line shows the change in cell viability over time with the CS1 chiller function set to 4°C and stirrer on. The green line shows cell viability over time without the CS1 at room temperature.
Conclusion
The combination of gentle, low-pressure sorting with the WOLF G2 Cell Sorter and the temperature control and interval stirring functions of the CS1 Chiller-Stirrer provides an ideal solution for reducing cell stress and maintaining cell viability for certain cell types while allowing for a fully automated cell sorting process.
For more information, visit nanocellect.com or email [email protected].
References
1. Yu, N. H., Chun, S. Y., Ha, Y. S., Kim, H. T., Kim, D. H., Kim, J.,…& Kwon, T. G. (2018). Optimal stem cell transporting conditions to maintain cell viability and characteristics. Tissue Engineering and Regenerative Medicine, 15, 639-647.
TCN-012
