Single-cell Sorting of T-Cells from a Heterogenous Population of Lyophilized Cells
Introduction
The WOLF Cell Sorter, developed by NanoCellect, is a novel microfluidic-based cell sorter. At less than 2 cubic feet, the WOLF is powered by a 488nm laser and can detect GFP, viability dyes and fluorescently labeled antibodies. Unlike traditional cell sorters, the WOLF is an easy-to-use system that can sort 300 cells/sec without exerting stress on the cells or creating hazardous aerosols. This is especially important for sorting fragile cell types and maintaining high cell viability. In addition to the WOLF, NanoCellect also offers the N1 Single Cell Dispenser that connects directly to the WOLF. The N1 can dispense 1 to 100 cells in a 96 or 384 well plate enabling use for several downstream applications such as cell line development, antibody discovery and single cell next-generation assays.
Veri-Cells™ are lyophilized human cells developed by BioLegend. Designed for long term stability, a scatter profile similar to fresh cells, and validated with more than 150 markers, Veri-Cells™ can be used to monitor data quality and aid with reproducibility in multi-center and longitudinal studies. In addition, these cells can be used in substitution of precious samples for routine checks in your experimental setup. Although not meant to replace specific samples when doing antibody titrations or instrument optimization, they can definitely help check or verify reagent and instrument performance. Furthermore, every lot of Veri-Cells™ is analyzed with a select number of phenotyping markers to ensure that the cell populations/marker staining is within the expected frequency, and reference values are reported with the cells Certificate of Analysis. Here, we demonstrate that Veri-Cells™ serve as a valuable tool for demonstrating the efficiency and precision of the WOLF and N1.
CD4 and CD8 T Cell Bulk Sorting with Veri-Cells™ PBMCs
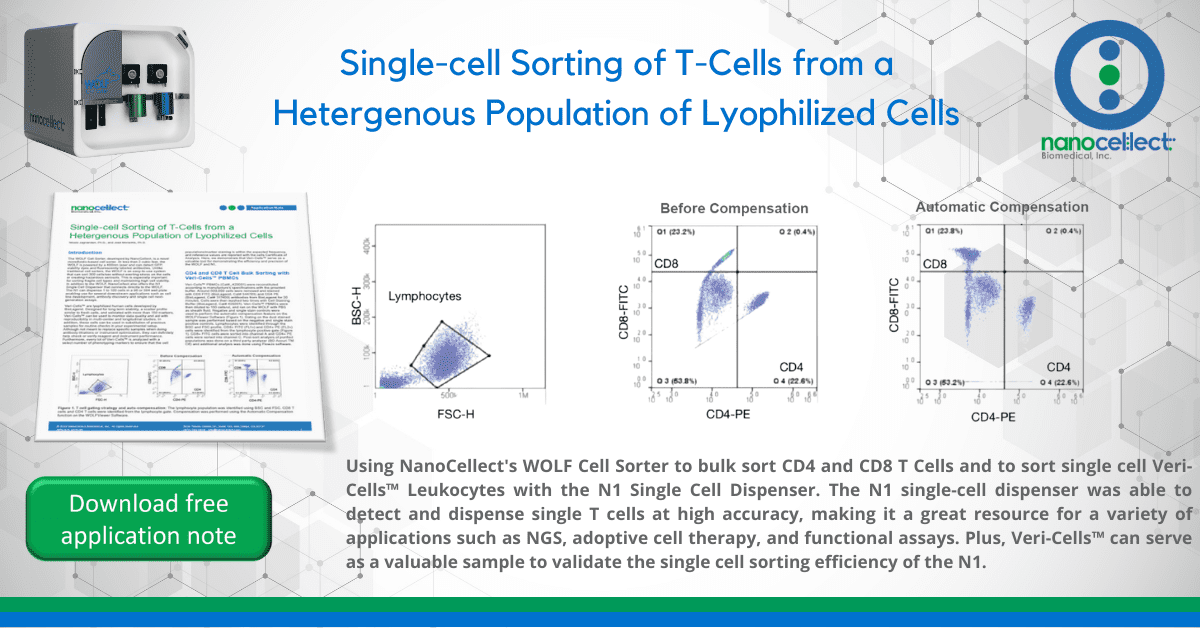
Veri-Cells™ PBMCs (Cat#_425001) were reconstituted according to manufacture’s specifications with the provided buffer. Around 500,000 cells were removed and stained with CD8 FITC (BioLegend, Cat# 344703) and CD4 PE (BioLegend, Cat# 317409) antibodies from BioLegend for 20 minutes. Cells were then washed two times with Cell Staining Buffer (BioLegend, Cat# 420201). Veri-Cells™ PBMCs were then diluted to 150 cells/uL and ran on the WOLF with PBS as sheath fluid. Negative and single stain controls were used to perform the automatic compensation feature on the WOLFViewer Software (Figure 1). Gating on the dual stained
sample was performed based on the negative and single stain positive controls. Lymphocytes were identified through the BSC and FSC profile. CD8+ FITC (FL1+) and CD4+ PE (FL2+) cells were identified from the lymphocyte positive gate (Figure 1). CD8+ FITC cells were sorted into channel A and CD4+ PE cells were sorted into channel C. Post-sort analysis of purified populations was done on a third party analyzer (BD Accuri TM C6) and additional analysis was done using FlowJo software.
Figure 1. T cell gating strategy and auto-compensation: The lymphocyte population was identified using BSC and FSC. CD8 T
cells and CD4 T cells were identified from the lymphocyte gate. Compensation was performed using the Automatic Compensation
function on the WOLFViewer Software.
Results
After bulk sorting around 9,000 cells of each CD8+ and CD4+ T cells, purity levels of 94% and 90% were obtained, respectively (Figure 2). These results show that the WOLF Cell Sorter is able to sort two distinct populations at the same time and obtain high purity levels. In addition, these results show that Veri-Cells™ are compatible with the WOLF Cell Sorter and serve as a useful resource to demonstrate its ease-of-use and sensitivity.
Veri-Cells™ single cell sorting using the N1 Single Cell Dispenser
Veri-Cells™ Leukocytes were reconstituted, stained with CD3 PE-Cy5 (BioLegend, Cat# 300309) for 20 mins and washed two times with cell staining buffer (BioLegend, Cat# 420201). Around 300,000 cells were then diluted to 75 events per uL in PBS and ran of the WOLF with the N1 (Figure 3A). Lymphocytes were identified through the BSC and FSC profile and events were selected off a singlet gate to reduce the amount of multiplets. Then, the CD3+ population was sorted based on a histogram positive gate (Figure 3B). Three independent plates of CD3+ single cell were collected. Each plate had two wells that served as our focusing positive controls with 100 cells in each. All three plates were then stained with SYTOX green (Thermo Fisher Scientific, Cat# S7020) and imaged on a NYONE instrument (SYNENTEC).
Results
After analyzing 3 plates, we found that 80%, 83% and 71% of the wells had 1 cell in them, respectively (Figure 3C). Percentages were determined out of a total of 94 wells because 2 wells served as our positive controls. Despite the heterogeneous nature and small size of T cells, the WOLF is
Figure 2. CD8 T cells and CD4 T cells were sorted into
two different channels on the WOLF Cell Sorter. Post Sort
analysis was confirmed using the BD Accuri TM C6 and further
analyzed using FlowJo software. After sorting around 9,000
cells into each channel, purity levels of 94% CD8 T cells and
90% CD4 T cells were observed.
able to detect these events, and the N1 is able to dispense a single cell at high accuracy. This data further shows that Veri- Cells™ can serve as a valuable sample to validate the single cell sorting efficiency of the N1. Furthermore, these results show that the N1 can sort a single T cell that can then be used for various applications such as characterization based on next generation sequencing assays, adoptive cell therapy, and functional assays.
In conclusion, these results show that Veri-Cells™ are an excellent resource for demonstrating the robust capabilities of the WOLF cell sorter, and the N1 single cell dispenser. In addition, these results show the utility of an alternative, versatile sample type or verified control cells, for optimizing your experiments before using your own valuable sample on our platform.
Figure 3. Veri-Cells™ Leukocytes were stained with CD3 PE-Cy5 and (A) single cell dispensed using the WOLF and N1 (B) CD3+
cells were gated using the histogram graph on the WOLFViewer Software. (C) Single cells were counted on the NYONE instrument
(SYNENTEC). Plate 1, Plate 2 and Plate 3 had a single cell efficiency of 80%, 83% and 71% respectively.
APN-015
