Purification of Multiple Myeloma Plasma Cells with the WOLF Cell Sorter

Introduction
Plasma cells are antibody-secreting cells that represent 2-3% of cells in the bone marrow. They differentiate from B cells and play a critical role in fighting foreign antigens. Due to the small percentage of cells, this population is difficult to isolate and maintain viability. Multiple myeloma (MM) is cancer characterized by the uncontrolled clonal growth of plasma cells in the bone marrow and production of a monoclonal M-protein. Treatments for MM continue to improve, however, many patients continue to have multiple relapses and succumb to disease. Isolating and characterizing MM plasma cells (MMPCs) has become rapidly essential for classification and decision making in medical cases. MMPCs also make up a small population of the bone marrow and have been shown to be hard to enrich for. This has hampered the ability to perform the necessary experiments to gain and clear understanding of the pathogenesis of this disease. Advances in the pathogenesis of MM is critical to improving patient outcome. Here, we show that the WOLF is gentle enough to isolate MMPCs while maintaining viability.
Method
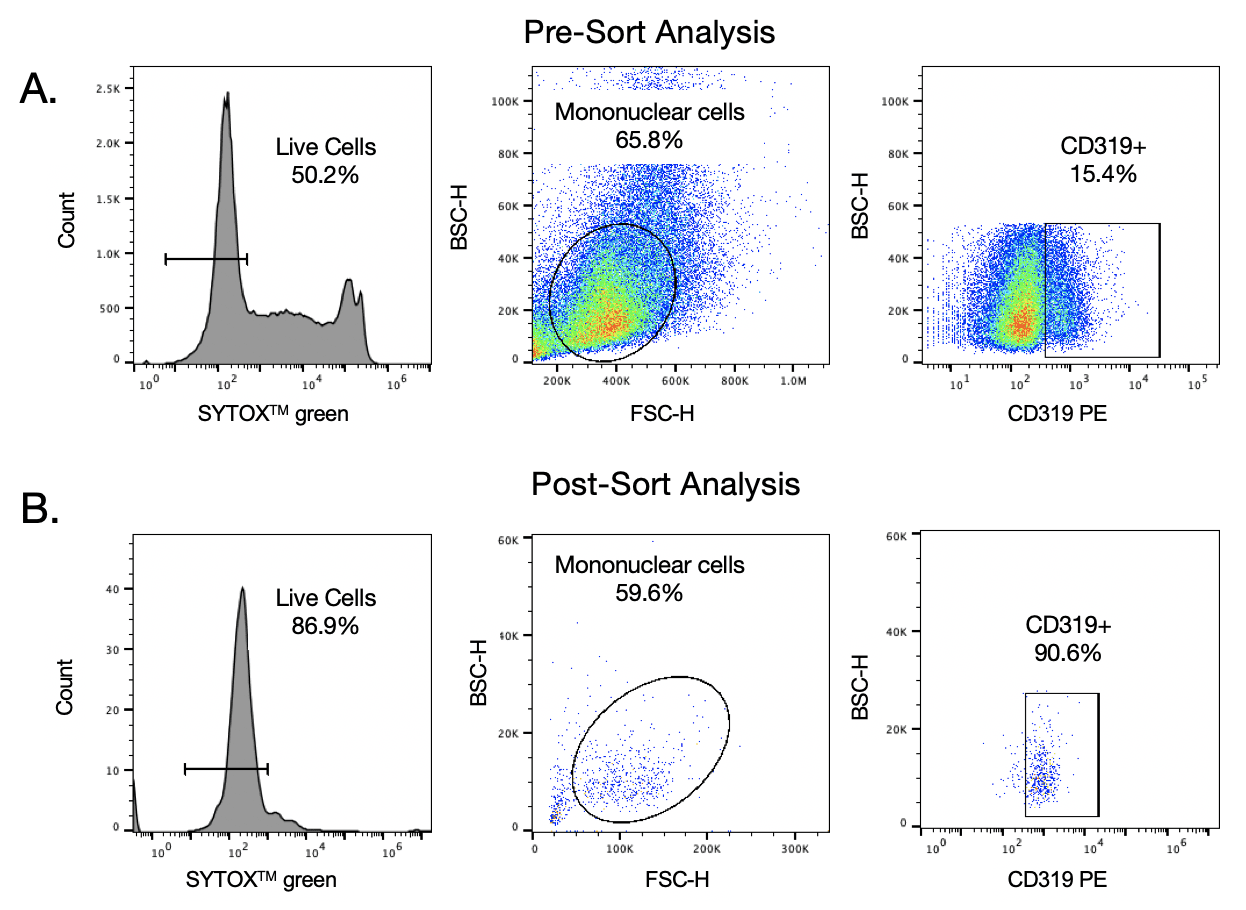
Frozen human mononuclear bone marrow cells were obtained from a MM patient (BIOVIT). Cells were then stained with CD319+ PE (BioLegend #331806) to identify MMPCs. These cells were then washed and diluted to 200 cells/uL and SYTOX green was added to determine cell viability. Cells were then analyzed and sorted using PBS+2%FBS as sheath. The sorting gate was based on the SYTOX green (live cells) negative population and CD319+ population (Figure 1A). 3,000 cells were then sorted into the A channel. Sorted cells were then stained again with SYTOX green to assess viability after being sorted through the WOLF. The BD Accuri TM C6 was used to analyze the purity of the MMPCs population.
Results
Pre-sort analysis on the WOLF showed that 50% of the cell population were viable and 15% of the live mononuclear cell population were MMPCs (CD319+) (Figure 1). After sorting, the WOLF was able to enrich the MMPC population to 90.6% and increase the live cell population to 87% (Figure 1B). These results show that the WOLF precise and gentle enough to sort MMPCs and maintain viability.
In conclusion, using the WOLF cell sorter is a robust platform to use in order to isolate rare, fragile cell types so that essential experiments can be done to understand life threatening diseases better.
APN-007

