Purification of Glia from Fresh and Fixed Mouse Brain Using the WOLF G2® Cell Sorter
Introduction
The brain is a heterogenous organ composed of different cell populations. Some of these cells are the neuroglia that include astrocytes, oligodendrocytes, and microglia. Neuroglia are known to have important functions in the brain such as modulation of homeostatic functions, myelination, nerve signal propagation, and responses to neural injuries1. The disruption in any one of these key functions is of importance in research because that can help explain mechanisms underlying a number of developmental and neurodegenerative diseases2.
Microglia typically account for less than 10% of the target cells in brain samples. Oligodendrocytes make up a target population of about 20% or higher, and astrocytes can make up between 17 to 61% of target populations3,4. Cell sorting can assist in these glial studies and enhance purification of individual cell types from diverse cellular populations, as found in the brain. In this work, the WOLF G2® Cell Sorter was used to sort astrocytes, microglia, and oligodendrocytes from fresh mouse tissue. In addition, a second sort was performed using fixed mouse brain due to the fact that researchers may not have access to fresh tissue or may have to flash-freeze the brain before the tissue can be processed.
Method
Brain Dissociation of Fresh Mouse Tissue
An adult mouse brain (C57BL/6, Crown Biosciences) was chopped with a scalpel into small pieces in a dish containing EBSS (Gibco 24010043) on ice. The tissue was centrifuged at 300 x g for 5 minutes and the supernatant was removed. 5 mL of enzyme mixture composed of 1X HBSS with calcium and magnesium (Gibco 14025092), 5% FBS (Genesee Scientific 25-514H), 1 mM HEPES (Gibco 15630080), 2 U/mL Dispase II (StemCell Technologies 07913), and 20 U/mL DNase I (Thermo Scientific 90083) were added to the brain pellet followed by resuspension by pipetting.
The sample tube was placed in a 37°C water bath. Every five minutes the sample was agitated 20 times since inversion causes the tissue to stick on other parts of the tube leading to sample loss during processing. After 15 minutes, the mixture was slowly triturated using a 200 µL and 1000 µL pipette tip to mix the sample by pipetting a total of six times ensuring that no bubbles were introduced. This agitation and trituration step was repeated for a total of three times. The sample was then filtered
through a 70 µm nylon mesh filter that had been pre-rinsed with cold sorting buffer composed of 1X HBSS without calcium and magnesium (Gibco 14170112), 1% BSA (Thermo Scientific 37525), and 2 mM EDTA (Invitrogen 15575020). The sample was then washed three times with sorting buffer and centrifuged at 300 x g for 5 minutes.
Removal of Myelin from Dissociated Fresh Brain
On the last wash, the brain tissue was resuspended with 1 mL of 1X RBC Lysis Buffer (BioLegend 420301), incubated for 3 minutes at room temperature, and washed with 9 mL of cold 1X HBSS without calcium and magnesium before centrifuging at 300 x g for 5 minutes. The supernatant was discarded to perform myelin removal.
Isotonic Percoll was prepared by diluting 9 mL of Percoll Plus (Cytiva 17-0891-02) with 1 mL of 10X HBSS without magnesium and calcium (Gibco 14185052). This isotonic solution was then further diluted with 1X HBSS without calcium and magnesium to create a 30% Percoll solution. The cell pellet that is now free of red blood cells was resuspended with 3 mL of 30% Percoll. The sample was centrifuged at 300 x g for 8 minutes with no brake. After centrifugation, the top layer containing myelin was discarded using transfer pipettes. The rest of the supernatant was removed until the cell pellet was reached. The pellet was then resuspended with excess sorting buffer and was washed two more times by centrifuging at 300 x g for 5 minutes each at 4°C. Lastly, the sample was resuspended with cold sorting buffer to perform cell staining.
Dissociation of Fixed Mouse Brain Tissue
One flash-frozen adult mouse brain (Rockland MS-T004) was placed in 1X zinc buffer (BD Biosciences 552658) overnight at 4°C. The following day, the brain was washed three times with sorting buffer and centrifuged at 300 x g for 10 minutes. After the last wash, the brain was placed on a weighing tray over ice and minced for 3 minutes. The tissue was transferred into a 15 mL tube containing approximately 3 mL of sorting buffer. Using a 1000 µL pipette tip, the sample was triturated by pipetting up and down 20 times and then left to settle on ice for 5 mins. After letting the sample settle, 2 mL of the cloudy supernatant was transferred to a new 15 mL tube and an additional 2 mL of sorting buffer was added to the remaining brain tissue to repeat trituration. The brain was triturated an additional five times while allowing the sample to settle in between to collect the cloudy supernatant. Once trituration was complete, the sample was filtered through a 70 µm mesh filter and centrifuged at 250 x g for 3 minutes each at 4°C to wash the cells twice using sorting buffer. The cells were resuspended with sorting buffer and used for staining.
Staining of Fresh and Fixed Mouse Brain Cells
The cells were blocked for 10 minutes on ice with TruStain FcX (anti-mouse CD16/32) antibody (BioLegend 101319). For the fresh mouse cells, one aliquot was stained with ASCA-2 PE (Miltenyi Biotec 130-123-284) and O4 APC (Miltenyi Biotec 130-118-978) and another aliquot was stained with ASCA-2 PE and CD11b APC (BioLegend #101212). The fixed mouse sample cells were stained with ASCA-2 PE only. Both fresh and fixed cells were incubated for 30 minutes at 4°C in the dark followed by washing. After washing, the samples were diluted to a final concentration of 3.0 x 105 cells/mL and filtered twice through a 40 µm cell strainer prior to analysis. In addition, the fixed cells were stained with DAPI (Invitrogen R37606) prior to sorting.
Bulk Cell Sorting and Result Analysis
The WOLF G2® with the red laser configuration (488/637 nm) was used to sort the fresh tissue cell samples. From the first aliquot that was stained with ASCA-2 and O4, ASCA-2+ cells were sorted into one channel to purify astrocytes and O4+ cells were sorted on the other channel to purify oligodendrocytes. The second sample that was stained with ASCA-2 and CD11b, had ASCA-2+ cells sorted into one channel to purify astrocytes once again and CD11b+ cells were sorted onto the second channel to sort microglia. Meanwhile, the fixed mouse sample consisted of sorting DAPI+ASCA-2+ cells using the WOLF G2® with the violet laser configuration (405/488 nm). The analysis and sorting was performed at 4°C and compensation was used to correct any potential spillover into adjacent fluorescent channels.
Approximately 50,000 astrocytes, microglia, and oligodendrocytes were sorted into collection tubes containing sorting buffer. Post-sort samples were spun down at 125 x g for 5 minutes to remove excess supernatant and then resuspended in at least 200 µL of sorting buffer to concentrate the sample. The samples were then re-analyzed to measure post-sort purity. Additionally, post-sort viability of the astrocytes from fresh mouse tissue was also measured after two hours of sorting by staining the sample with 7-AAD (BioLegend 420403) and compared to the pre-sorted sample and a control sample composed of 25% heat-killed cells and 75% live cells.
Results
Post-Sort Purity
The WOLF G2® Cell Sorter was capable of purifying astrocytes, microglia, and oligodendrocytes isolated from fresh brain and astrocytes from fixed brain. Zinc-fixation was used instead of traditional methods since it has been demonstrated that this method can be less toxic to some enzymes of interest, results in a higher yield of cells needed for sorting, and can help maintain RNA quality5,6.
Before sorting, the starting target populations from fresh mouse brain for astrocytes was 25.8%,15.6% for microglia, and 40.7% for oligodendrocytes. Post-sort purity of the fresh mouse tissue for astrocytes, microglia, and oligodendrocytes was of 92.7%, 95.7%, and 93.2% respectively. Astrocytes from fixed mouse tissue were enriched from 58.2% to 81.5% after sorting (Figure 1).
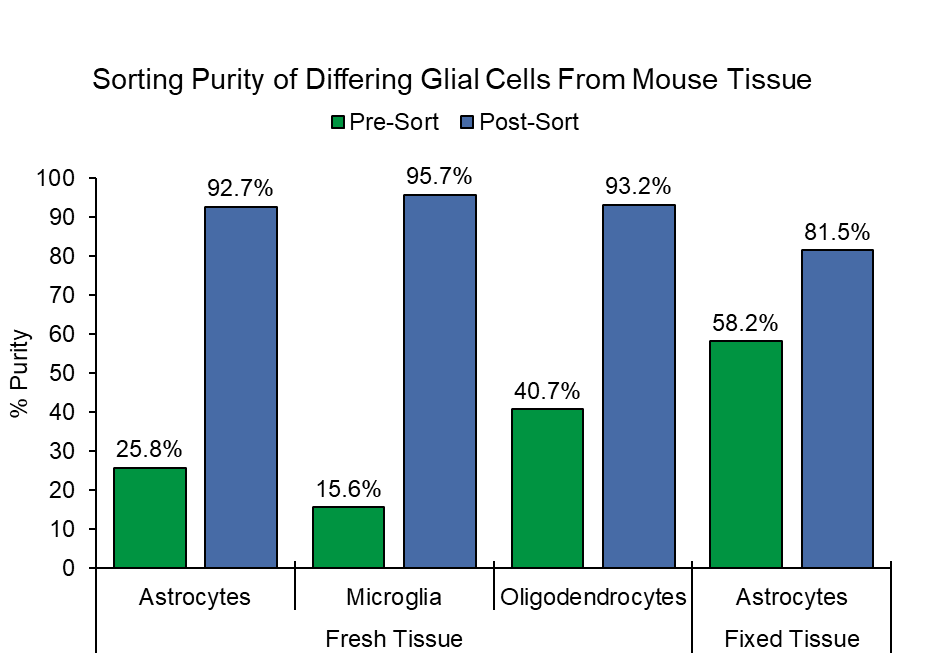
Figure 1. Purity of Astrocytes, Microglia, and Oligodendrocytes from Fresh Tissue and Astrocytes from Fixed Tissue: Two different sorts were performed with mouse tissue where astrocytes and microglia were sorted from a sample stained with ASCA-2 PE and O4-APC. This was followed by a second sort using a sample stained with ASCA-2 PE and CD11b-APC to sort astrocytes and oligodendrocytes. Sorting of the cells resulted in a greater than 90% purity. Meanwhile, astrocytes from fixed brain had a post-sort purity that was greater than 80%.
Pre-sort, the fixed brain tissue sample showed notable amounts of astrocyte debris (Figure 2A). After sorting the DAPI+ASCA-2+ cells, the debris was highly depleted leaving behind mostly purified astrocytes (Figure 2B).
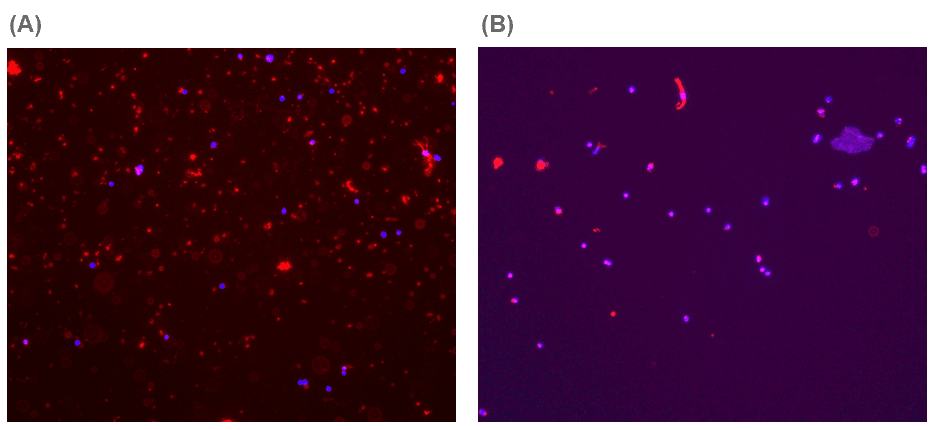
Figure 2. Representative Image of Fixed Tissue Before and After Cell Sorting Stained with ASCA-2 (red) and DAPI (blue): (A) When analyzing the fixed tissue before sorting, one can visualize that the astrocyte marker can stain debris, which highlights the importance of adding the DAPI-marker to differentiate between debris and actual cells. (B) Post-sort of the cells shows that the debris was depleted with sorting and that the sorted cells were mostly astrocytes based on the merging of DAPI and ASCA-2 (purple). Cells imaged on the ECHO Revolve at 4x magnification.
Post-Sort Viability from Fresh Mouse Tissue
Viability of the sorted astrocytes from fresh brain remained unaffected when comparing it to the pre-sorted sample where more than 90% of the cells were still alive. Pre-sort, cell viability was 92.5% and increased to 98.9% post-sort. A live/dead control mixture was 73.6% viable (Figure 3). This highlights that gentle sorting is beneficial to maintain cell viability.
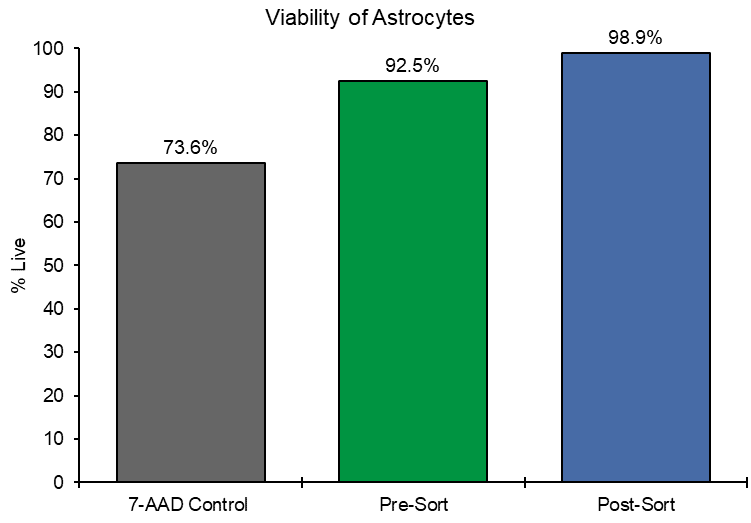
Figure 3. Viability of Astrocytes: 7-AAD was used as a viability stain of astrocytes isolated from fresh brain tissue. Viability is shown as a percentage of live cells in the control sample as well as before and after sorting demonstrating that astrocytes remained viable after sorting.
Conclusion
In conclusion, users can use fresh and fixed mouse brain to isolate target cell populations including astrocytes, oligodendrocytes, and microglia, allowing access to broader tissue availability for downstream assays such as single-cell RNA sequencing, without having to rely on using nuclei. With the WOLF G2® Cell Sorter, users can enrich astrocytes, oligodendrocytes, and microglia from a heterogeneous brain sample at a purity of >90% while maintaining high cell viability from fresh tissue.
For more information, visit nanocellect.com or email [email protected]
References
1. Domingues HS, Portugal CC, Socodato R and Relvas JB (2016) Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 4:71. doi: 10.3389/fcell.2016.00071
2. Agalave NM, Lane BT, Mody PH, Szabo-Pardi TA, and Burton MD (2020) Isolation, culture, and downstream characterization of primary microglia and astrocytes from adult rodent brain and spinal cord. Journal of Neuroscience Methods. 340: 108742. https://doi.org/10.1016/j.jneumeth.2020.108742
3. Valério-Gomes B, Guimarães DM, Szczupak D, Lent R. The Absolute Number of Oligodendrocytes in the Adult Mouse Brain. Front Neuroanat. 2018 Oct 30;12:90. doi: 10.3389/fnana.2018.00090
4. Garland EF, Hartnell IJ and Boche D (2022) Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 16:824888. doi: 10.3389/fnins.2022.824888
5. Martin D, Xu J, Porretta C, Nichols CD (2017). Neurocytometry: Flow cytometric sorting of specific neuronal populations from human and rodent brain. ACS Chem Neurosci. 8(2): 356–367. doi:10.1021/acschemneuro.6b00374.
6. Abshair M, Adams D, Bergeron A, Brundage K, Clise-Dwyer K, Cochran M, Del Rio Guerra R, Holmes L, Lood N, Meyer M, Niziolek Z, Saluk A, and Thornton S (2019). A Multi-Core Study on How Different Fixation Methods Prior to Sorting Impact the Purity, Quality, and Yield of RNA From Sorted Cells. Poster presented at: ABRF 2019 – The Association of Biomolecular Resource Facilities; April 23, 2019; San Antonio, TX.
APN-039
