Gently Sort Digested Skin Tissue Cells on the WOLF
Introduction
Obtaining viable single-cell suspensions from tissues is often challenging due to harsh mechanical dissociation and enzymatic digestion methods. As a result, substantial numbers of cells are often lost and/or have stress responses that can influence downstream growth or phenotypes, such as immune activation. Lysed cells and extracellular material contribute to contaminating debris in cell samples and can result in aggregation. Downstream applications that rely on a pure cell population can be limited by these contaminating debris. Fluorescence-activated cell sorting can be a valuable tool for sample clean up that isolates a target cell population and reduces debris. While conventional droplet-based cell sorters can enrich cells, their use of high-pressure fluidics can further stress or lyse sensitive cells. The WOLF® Cell Sorter is a gentle microfluidic cell sorter that applies extremely low pressure (>2 psi) to cells. With 5 detection parameters, the WOLF can gently and effectively enrich a target population while avoiding dead cells and debris. This application note demonstrates how the WOLF can successfully isolate a dynamic cell type, skin-resident T cells, while maintaining high viability.
Method
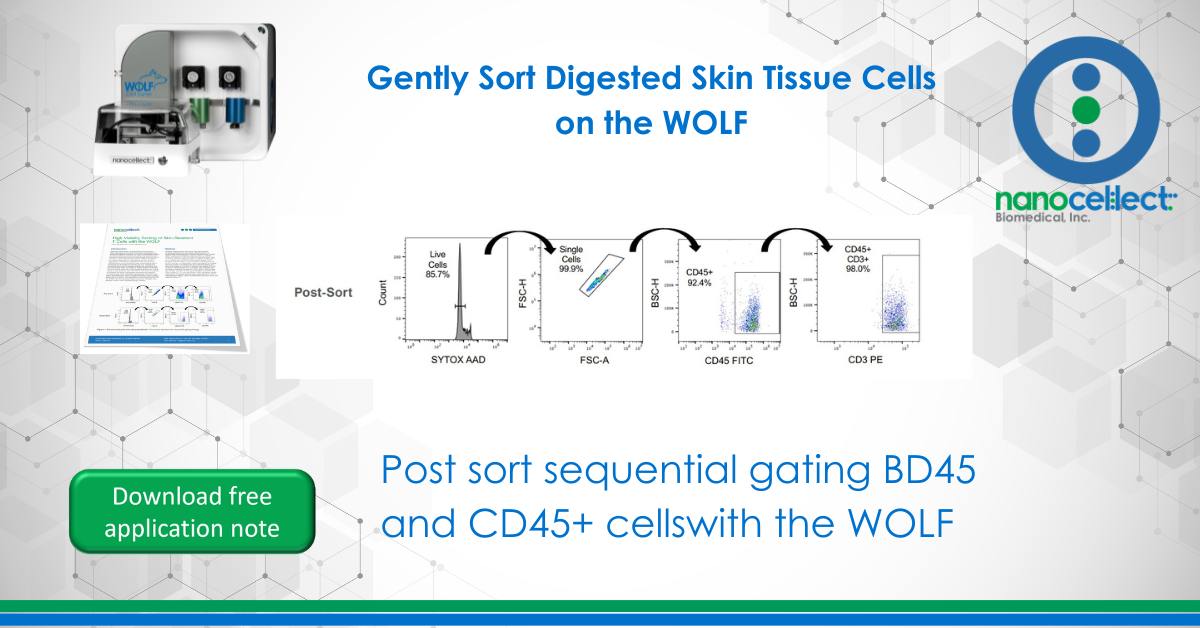
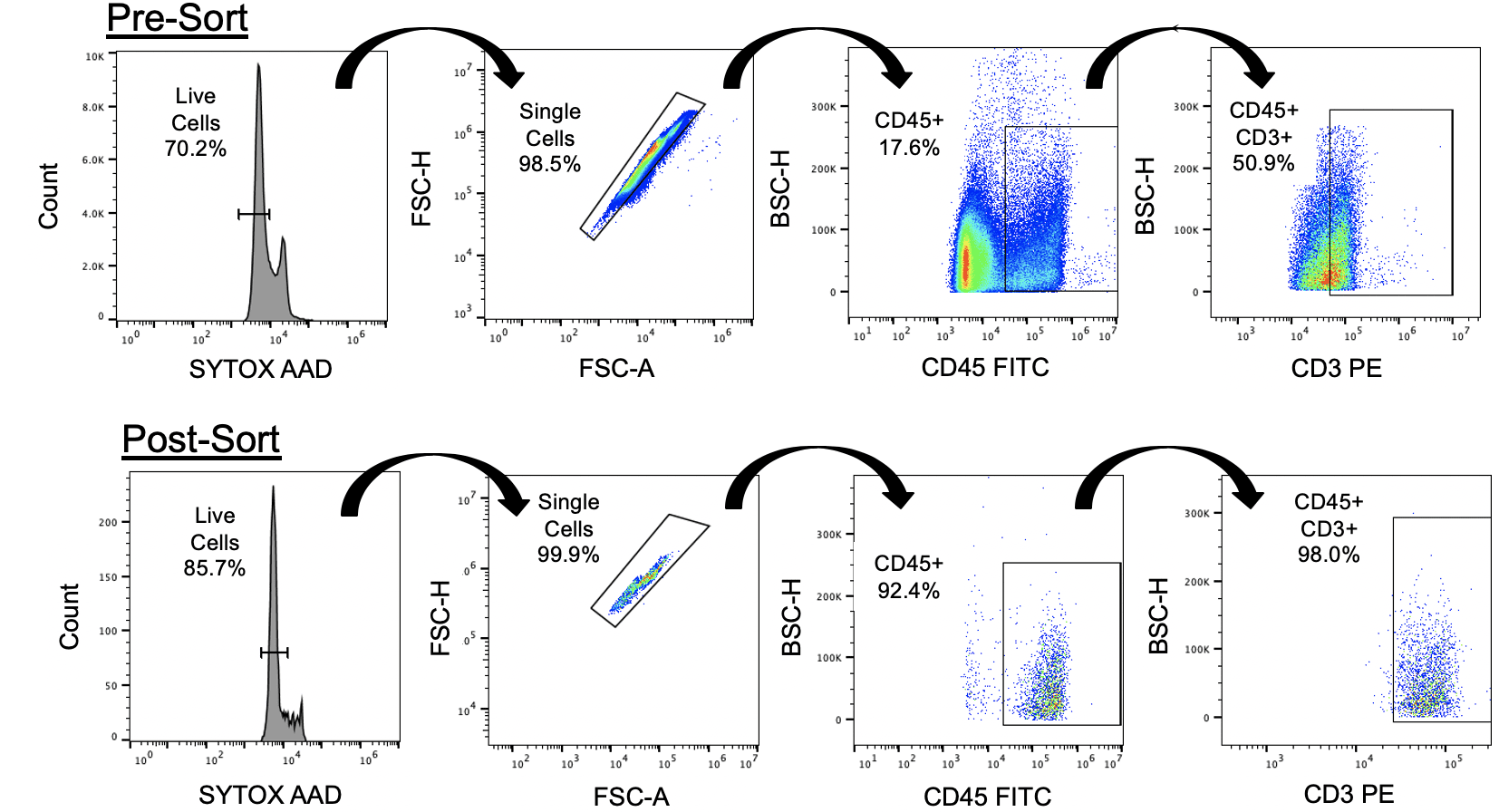
Digested healthy human skin tissue cells were isolated by standard methods and provided to NanoCellect by a collaborating pharmaceutical company (He et al 2016). CD45+ CD3+ T cells were identified by staining with 5 µL of FITC CD45 FITC and 5 µL CD3 PE in 90 µL of staining buffer. Cells were then washed in staining buffer and diluted to 300 cells/ µL. A viability dye, SYTOX™ AADvanced™ Ready Flow™ Reagent (ThermoFisher), was then added at 2 drops per mL prior to sorting. A compensation matrix was applied to mitigate spectral overlap. Sort gates were set to exclude debris and dead cells based on the ratio of forward side scatter (FSC) to SYTOX™ AADvanced™ (FL-3 channel), and doublets were avoided by gating for singlets on a FSC-Width / FCS-Height plot. Finally, activated T cells were identified as CD45+ and CD3+ (Figure 1). 7 x 103 live CD45+ CD3+ cells were sorted. A portion of the sorted sample was re-stained with SYTOX™ AADvanced™ Ready Flow™ to determine post-sort purity and viability (Figure 1).
Results
Prior to sorting only 70.2% of the cells were viable, and 8.8% of these live cells were double-positive for CD45 and CD3. After sorting, the proportion of live cells was increased to >85% and >90% of the live population was both CD45+ and CD3+. As a result of using gentle cell sorting, the target population of viable CD45+ CD3+ cells was increased by >10x while contamination from debris was reduced by half.
Conclusion
Obtaining high-viability from a specific target population from primary tissue is challenging due to high levels of cell death and contaminating cellular debris. Furthermore, conventional cell sorting methods that rely on high-pressure droplet-sorting technologies can further reduce viability. Here we show that low-pressure microfluidic-based cell sorting can effectively enrich sensitive cells, such as skin-resident T cells to levels that are valuable for downstream assays, such as single-cell RNA-seq.
References
He X, de Oliveira VL, Keijsers R, Joosten I, Koenen HJ. Lymphocyte Isolation from Human Skin for Phenotypic Analysis and Ex Vivo Cell Culture. J Vis Exp. 2016;(110):e52564. Published 2016 Apr 8. doi:10.3791/52564
APN-011
