High-Pressure vs. Microfluidic: The Case for Gentle Cell Sorting

The idea to combine microscopy and sheath fluid to focus cells hydrodynamically was first dreamed up back in 1953 by Crosland-Taylor in his Nature publication. Seven years later, the first cell sorter was developed in Los Alamos, launching the future of fluorescence-activated cell sorting, commonly known as FACS.
Traditionally, cell sorters have been built for processing samples at high-speed, which over time has been shown to cause problems (which we’ll dive into throughout this blog post) for some applications. In the 2000s, microfluidics-based cell sorters entered the scene, offering a new option for labs.
Why Microfluidics?
When running a cell sorting experiment, have you ever asked yourself one of these questions?
-
-
-
- Why do my cells die after sorting?
- Why are my cells activated?
- If I cleaned the sample line, why am I still seeing carryover?
- Why did my cell sort purity and efficiency decline?
- Why don’t my cells seem to grow well post-sort?
- Why did I get contamination in my sorted sample, even after decontaminating the fluidics?
-
-
If you have, you may be dealing with complications caused by traditional cell sorters. While high-pressure sorting means faster speeds, that advantage comes with other sacrifices that can negatively impact your experiments.
Traditional, High-Pressure vs. Gentle, Microfluidic Cell Sorting
Let’s look at a few areas with key differences between high-pressure and microfluidic-based sorting…
Pressure Over Time
The traditional electrostatic or droplet-based cell sorters expose cells to stress at multiple points throughout their journey through the cell sorter. One of the most significant is the passage through the flow cell.
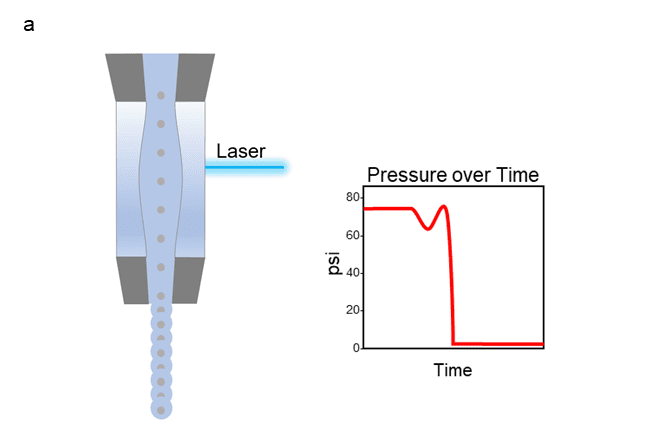
Figure 1 shows both a cuvette (a) and jet-in-air (b) style cell sorter along with corresponding psi levels as cells move through the flow cell portion of the instrument. For both types, the cells enter the flow cell at a very high pressure (typically anywhere from 20 to 300 psi depending on the instrument), which then rapidly drops as they exit through the nozzle opening. This dramatic change in pressure causes decompression shock and shear stress, leading to damaged cells with poor cell health and viability. Even if the cell survives, the stress of sorting can alter many aspects of the cell’s phenotype, such as proliferation, activation status, morphology, metabolism, and even gene expression.
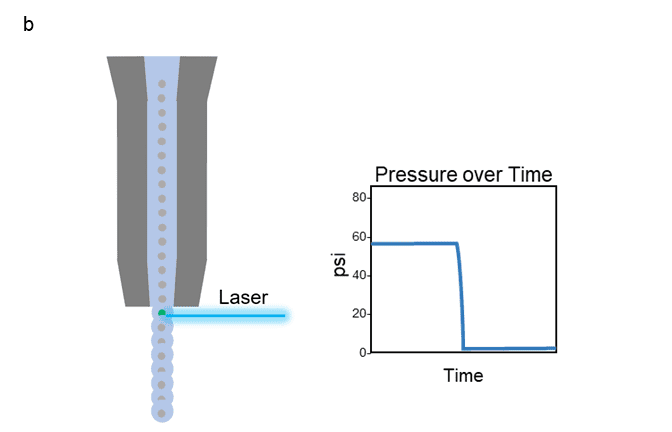
Figure 1: Depiction of the path a cell takes as it travels through the flow cell portion of a cuvette style cell sorter (a) or jet-in-air style cell sorter (b), with graph of corresponding pressure over time.
Our Sr. Product Manager, Mandana Farhadi, describes it well with a metaphor in her webinar, “…when two vehicles are traveling down a road at high speeds, even if their speeds are not very different from one another, an impact at that velocity can cause a lot of damage”.
In contrast, Figure 2 shows an example of a cell’s path through NanoCellect’s microfluidic cartridge. The change in psi is virtually non-existent, and the overall level remains below 2 psi. Maintaining this ultra-low level of pressure achieves a very gentle sort, reducing the chances of decompression shock and shear stress impacting the cells.
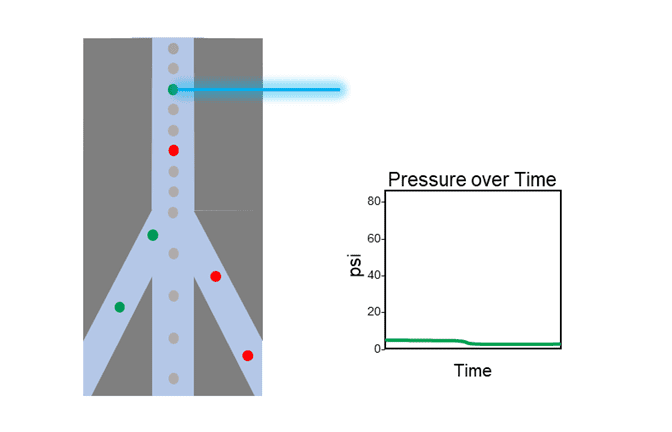
Figure 2: Depiction of the path a cell takes as it travels through the cartridge of a microfluidic cell sorter, with graph of corresponding pressure over time.
Sheath Fluid Options
High-pressure systems are usually limited to PBS (phosphate-buffered saline) buffers as the only sheath fluid option. This limits the pH to a level of about 7.4, which may not be best for some cell types.
For example, unicellular organisms and protoplasts require specialized buffers to maintain osmotic regularity. Microfluidics-based sorters generally allow for alternative sheath options. The microfluidic cartridges used with the WOLF systems accommodate different types of sheath fluid, allowing for optimized cell conditions when making its way through the cell sorter.
Sterilization
Traditional cell sorters are bulky instruments with large footprints, making use of biosafety cabinets challenging and costly. The large fluidics containers such as sheath and waste tanks require complex and routine maintenance, making sterilization a hassle. Sample lines must be cleaned between sample runs, which can result in cross contamination since the fluidics path is not disposable.
A microfluidic cell sorter, such as the WOLF G2, is compact in size and can easily fit inside any standard biosafety cabinet. The sterile, disposable microfluidic cartridge has a fully contained fluidics path, providing confidence in sterility of the sorted samples.
Gentle Sorting, Healthy Cells, Better Science
Now that we’ve highlighted some of the key differences between high-pressure and microfluidic cell sorting, how does it apply as a better option in the lab? Sample prep for downstream genomics, maximizing cell viability and clone outgrowth for cell line development, and antibody discovery research for pre-clinical characterization are just a few applications that can benefit from gentle cell sorting.
Curious about real-life examples? Check out our scientific publications page where the WOLF or WOLF G2 was used across many different application types, or contact us for more information.