Post-Sort Sample Recovery on the WOLF Cell Sorter
Introduction
Traditional droplet-based fluorescence activated cell sorters are able to isolate pure cell populations at high speeds. This often comes, however, at the cost of reduced cell recovery. Cell death induced from the high-pressure droplet-sorting mechanism, cells moving out of the droplet, and high abort rates1 can all contribute to low cell recovery rates. It has been reported that traditional cell sorters can lose up to 70% of their sorted cell population.2,3,4This can be a challenge when working with rare cell populations and limited samples. Furthermore, cells adhering to the fluidics system or the collection tube, as well as post-sort centrifugation can contribute to a lower sample recovery. In contrast to traditional cell sorters, the WOLF Cell Sorter uses low pressure and gentle mechanical microfluidic sorting to efficiently isolate targeted cell populations. In this technical note, we measured the sample recovery rate from the WOLF Cell Sorter.
Method
Fluorescent 15 μm Dragon Green beads (Bangs Labs, #FSDG009) and live adherent HEK293T cells (European Collection of Authenticated Cell Cultures, #12022001) were prepared at a concentration of 200-300 events/μL. Each sample type was then set to sort 25,000 and 100,000 events based on their scatter plot properties using a singlets gate. This was repeated 5 times for each 25,000 and 100,000 bead or cell sort. This procedure was then repeated at a higher sample concentration of 500-600 events/μL. Each sorted sample was then centrifuged at 350 x g for 5 minutes. Without disturbing the pellet, the excess volume was removed to leave 100 μL of buffer inside the collection tube. Each sample was then resuspended and counted on the Countess II Automated Cell Counter (Applied Biosystems™) twice. These cell counter values served as a reference and were then compared to the number of events the WOLF calculated to determine the percentage of cells recovered.
Results
At a sample concentration of 200-300 events/μL, the average recovery for the 25,000 event sort was 73±0.9% for both bead and HEK293T cells. While for the sort of 100,000 events, there was higher sample recovery of 86±0.9% for both cells and beads (Figure 1A). There was little effect of sample concentration as indicated by sorting samples on the WOLF at a higher concentration of 500-600 events/μL. The average recovery for the 25,000 and 100,000 event sorts were 72.16±1.6% and 88±5.4%, respectively, for both cells and beads (Figure 1B).
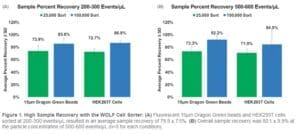
Conclusion
In both the high and low concentrations, there was an increase in recovery of about 10% in the 100,000-event sorts, relative to the 25,000-event sorts. In all cases, these recovery rates are substantially higher than those reported for conventional droplet-based sorters. In addition, there was only a 10% difference in sample recovery between sorting at lower or higher concentration. Overall, the average recovery was 80%±8.1% across all sorted samples. The WOLF’s gentle microfluidic sorting mechanism, and low shear stress, most likely contributed to the high cell recovery rates relative to droplet sorting. In addition, these experiments further demonstrate that the WOLF can be beneficial when sorting limited samples in order to maximize cell recovery. Furthermore, these experiments provide a guideline to calculate the number of cells that need to be sorted for downstream assays when using the WOLF.
