Improved single cell cloning using microfluidic cell sorting
Abstract
Cell line development has been an essential step in finding new drugs, toxicity studies, and in-vitro testing. The process is also necessary for antibody production, cell therapy, and gene editing.
Commonly, mammalian cells have been used, but the usage of other systems such as bacteria, yeast, and plants have started to emerge. Single-cell cloning requires a cell line of interest to originate
from a single cell to decrease heterogeneity that may lead to changes in cell outgrowth or issues such as discrepancies in protein production. One traditional method used is limiting dilution. However, the method is time-consuming, results in low single-cell efficiency, and has shown that it requires additional rounds of cloning to ensure that the colony has single-cell origin.
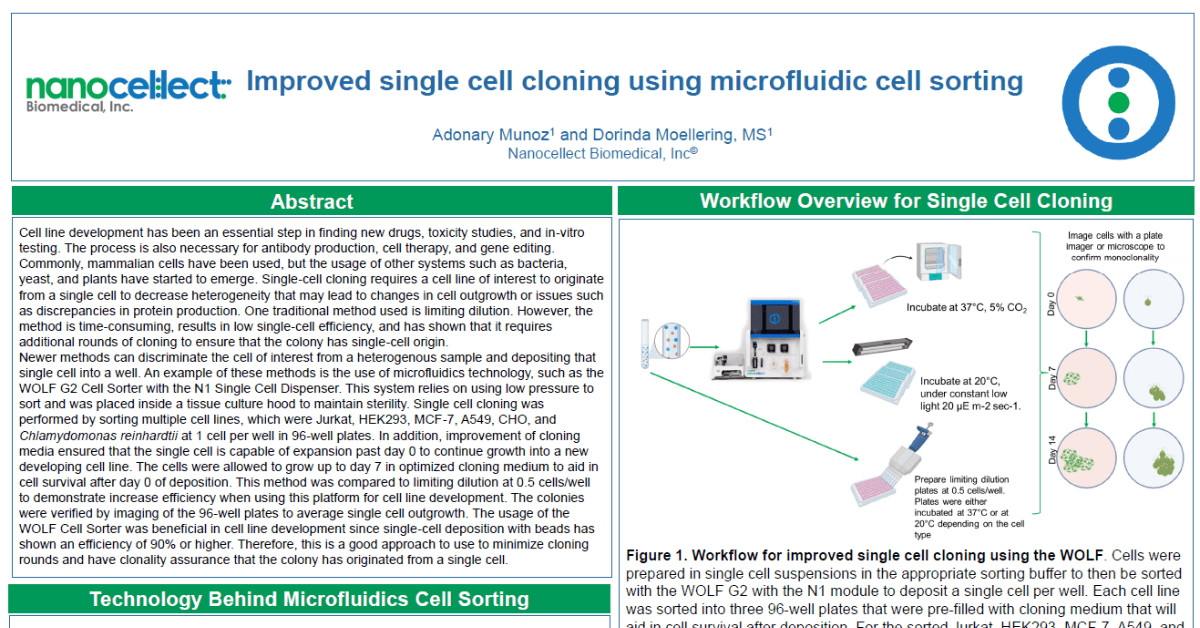
Newer methods can discriminate the cell of interest from a heterogenous sample and depositing that single cell into a well. An example of these methods is the use of microfluidics technology, such as the WOLF G2 Cell Sorter with the N1 Single Cell Dispenser. This system relies on using low pressure to sort and was placed inside a tissue culture hood to maintain sterility. Single cell cloning was performed by sorting multiple cell lines, which were Jurkat, HEK293, MCF-7, A549, CHO, and Chlamydomonas reinhardtii at 1 cell per well in 96-well plates. In addition, improvement of cloning media ensured that the single cell is capable of expansion past day 0 to continue growth into a new developing cell line. The cells were allowed to grow up to day 7 in optimized cloning medium to aid in cell survival after day 0 of deposition. This method was compared to limiting dilution at 0.5 cells/well to demonstrate increase efficiency when using this platform for cell line development. The colonies were verified by imaging of the 96-well plates to average single cell outgrowth. The usage of the WOLF Cell Sorter was beneficial in cell line development since single-cell deposition with beads has shown an efficiency of 90% or higher. Therefore, this is a good approach to use to minimize cloning rounds and have clonality assurance that the colony has originated from a single cell.
PST – 010
