How to Conduct an Antibody Titration
Three Ways an Antibody Can Bind to a Cell
The first is the binding of the active site (FAb) to the target antigen.
The second is Fc Receptor-mediated binding, this occurs on cells that express the Fc Receptor including B-cells, NK cells, neutrophils, eosinophils, basophils and mast cells. This is a specific binding, but not what we typically want.
The third type of binding is non-specific or low affinity binding, where the antibody binds to some off-target. This last binding is a concern, as it can reduce the sensitivity of the assay by increasing the spread of the negatives.
Conducting an Antibody Titration Experiment
To reduce this loss of sensitivity, flow cytometrists often conduct an antibody titration experiment using the antigen of interest and the target cells. In fact, it is recommended as one of the optimization steps that all researchers should perform. In this type of experiment, the researcher performs serial dilutions of the antibody, such as shown in Figure 1.
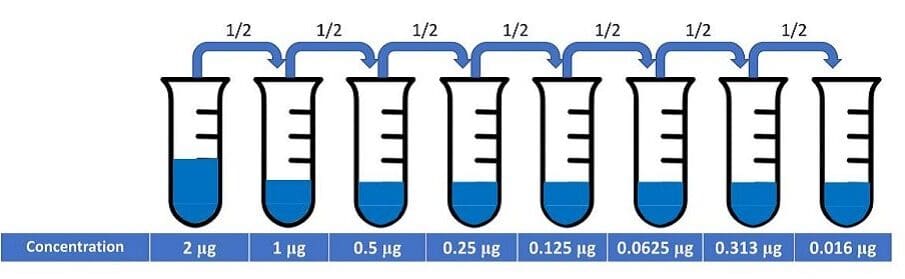
Figure 1: Serial dilutions of an antibody for a titration experiment.
When one performs this type of experiment, there are several important caveats. The first is to fix the time, temperature and staining volume using a known concentration of cells. Second is to make sure that the cells are stained in the same manner that the experiment will be performed – so if the cells will be fixed, so should the cells being tested.
Once the data is acquired on the flow cytometer, the analysis begins by gating the cells to identify the positive and negative population. Once the gating is completed, several statistics are extracted. From the positive gate, extract the median fluorescent intensity (MFI) and from the negative gate, extract the MFI and the robust standard deviation (rSD).
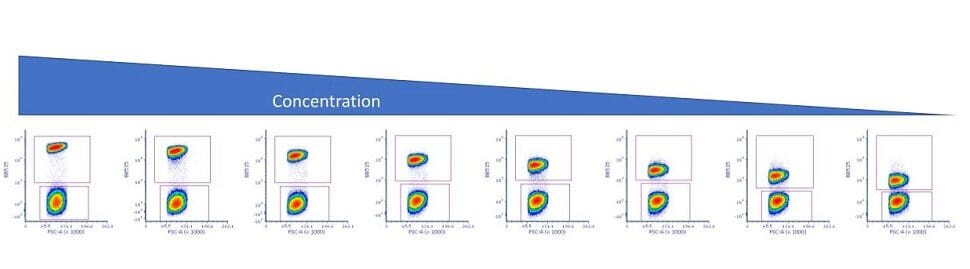
Figure 2: Titration data showing positive and negative gates.
Using this data, the staining index (SI) can be calculated. This is a unitless number that allows for easy comparison of the different concentrations. The SI calculation is shown in Figure 3.
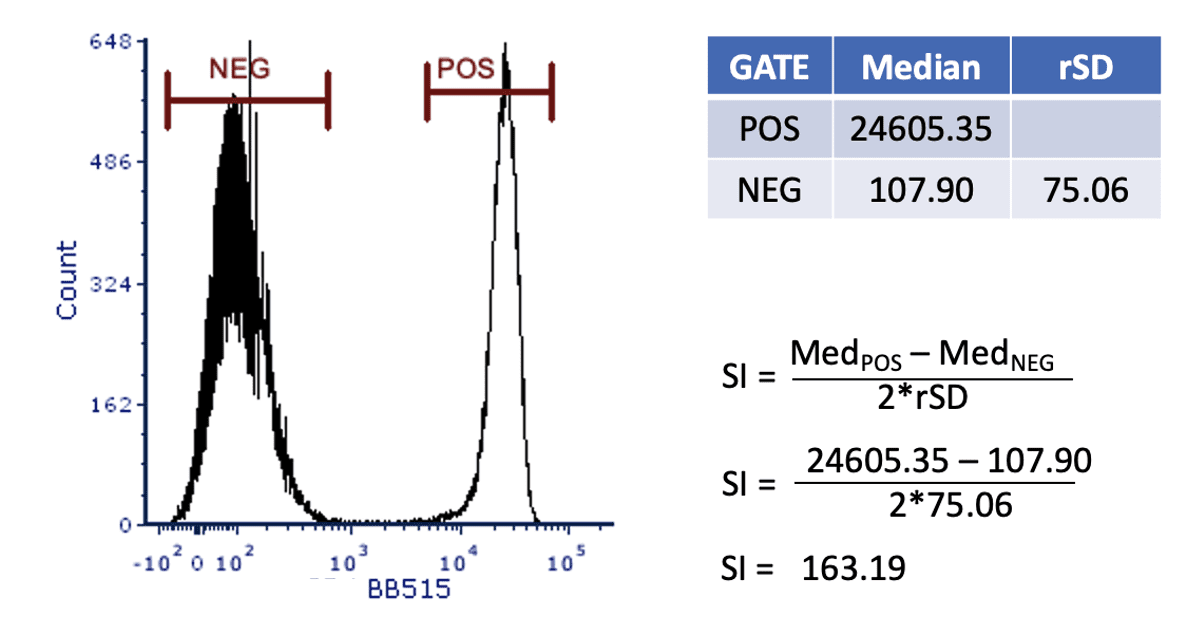
Figure 3: Calculating the staining index.
The final step in the process is to plot the SI versus the antibody concentration, as shown in Figure 4.
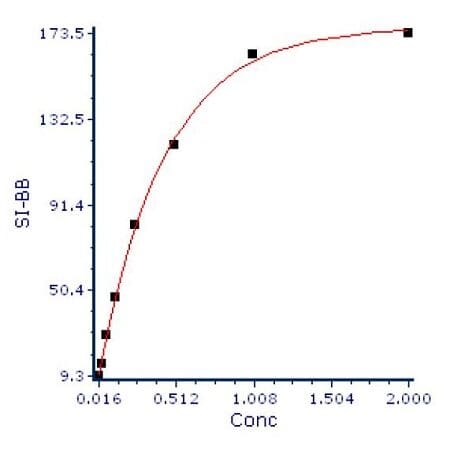
Figure 4: Ab concentration vs SI.
Analyzing Results
To interpret this graph, we look for the point on the curve where increasing concentration does not show a linear increase of the staining index. In this case, that point is somewhere between 0.5 and 1 μg of antibody. In this case, 0.75 μg would be a good concentration to use for this antibody.
The most important variable in staining is the final concentration of the antibody, so that if the calculation requires 1 μg of antibody in 50 μl final volume, it is reasonable to stain as many as 1×107 cells in that 50 μl volume. If you increase the volume to 100 μl, then you will need to increase the antibody volume to keep the concentration consistent. Consider staining 1×108 cells for a sorting experiment. If these were resuspended in 150 μl, one might increase the antibody concentration by a factor of 3. Of course, it is always worth testing and validating the staining before proceeding.
Finally, there is a trick that one can do to display all the titration values at the same time. Most analysis software allows for the concatenation of the datafiles. This is shown in Figure 5.
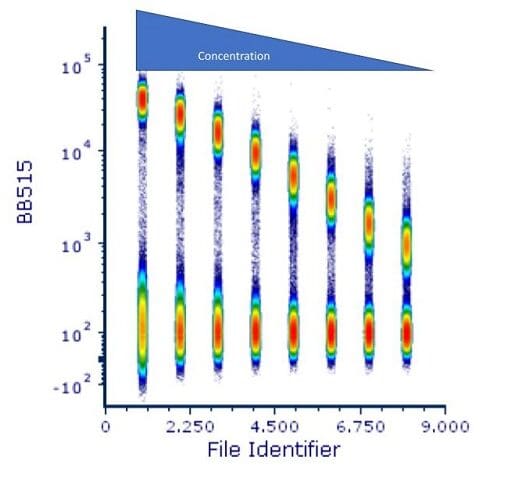
Figure 5: Concatenated titration files.
This is a great way to show the results of this and other experiments such as voltage optimization. It allows for the visualization of the spread of the data at the low end, while showing the decreasing positive signal as the antibody concentration decreases.
Antibody titration is a critical step in optimizing a flow cytometry experiment. It helps to ensure that the optimal concentration is used for staining, and minimizes the loss of resolution caused by the non-specific binding of the negative cells. It is essential for good results and is a flow cytometry best practice.