Sorting Single-Cell Suspension CHO Cells Optimized for High Outgrowth
Download PDF
Introduction
Suspension cell lines are critical for the scaled-up production of proteins and biologics intended for research and clinical use, respectively. Suspension cells producing biologic drugs need to be grown serum-free to avoid using inconsistent animal derived serums, having to use purification methods to eliminate serum proteins during downstream processing, and to avoid the potential of introducing harmful microbes, viruses or prions.1,2 In addition, cell line development and single-cell cloning have previously depended on inefficient methods such as limiting dilution or complex high-pressure cell sorters that negatively affect cell outgrowth.3,4 Moreover, low monoclonality rates can lead to genetic heterogeneity when producing cell banks or for antibody production.5 With the WOLF Cell Sorter and the N1 Single-Cell Dispenser, users can rely on an easy-to-use platform for suspension cell outgrowth that relies on serum-free preparation while maintaining high viability and monoclonality.
Methods
Suspension CHO-ES cells (Expression Systems, catalog #94-008F) were expanded and maintained in serum-free ESF SFM Mammalian Cell culture medium (Expression Systems, catalog # 98-001). Cells were prepared for single-cell sorting using the WOLF by diluting the cells in sample buffer composed of ESF SFM medium + 25 mM HEPES (Gibco, catalog #15630080). The sheath buffer was also composed of ESF SFM medium + 25 mM HEPES. Cells were prepared to have a final sorting concentration of 1 x 105 cells/mL. Suspension CHO-ES cells were sorted based on their scatter properties using a singlets gate without the use of viability dyes (i.e. label free).
The cells were sorted using the N1 Single Cell Dispenser into a total of 96 well dispenser plates that were pre-filled with 200 μL of optimized cloning medium. Optimized cloning medium was composed of 80% EX-CELL CHO cloning medium (Sigma, catalog # C6366) that was optimized with 6 mM GlutaMAX (Gibco, catalog # 35050061), 20% ESF SFM medium, 1X MEM Non-Essential Amino Acids (Gibco, catalog #11140050), and 1:80 ClonaCell-CHO ACF supplement (STEMCELL, catalog #03820). The testing conditions were repeated to complete a total of three WOLF Cell Sorter cartridges and nine 96-well plates. In addition, one WOLF Cell Sorter cartridge was used to perform the same sort, but cells were sorted into three 96-well plates that were pre-filled with 200 μL ESF SFM Mammalian Cell culture medium as a negative control. For all sorted plates, well D4 was spiked with the stock CHOES cells to aid in focusing when imaging the plates. The plates were stored in the incubator at 37°C, 5% CO2 for 14 days. On Day 7 and Day 14, the plates were imaged using the Celigo (Nexcelom) with only the brightfield channel to confirm monoclonality and outgrowth.
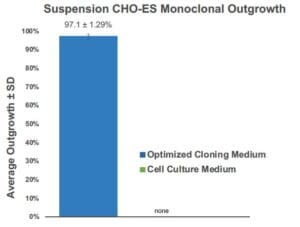
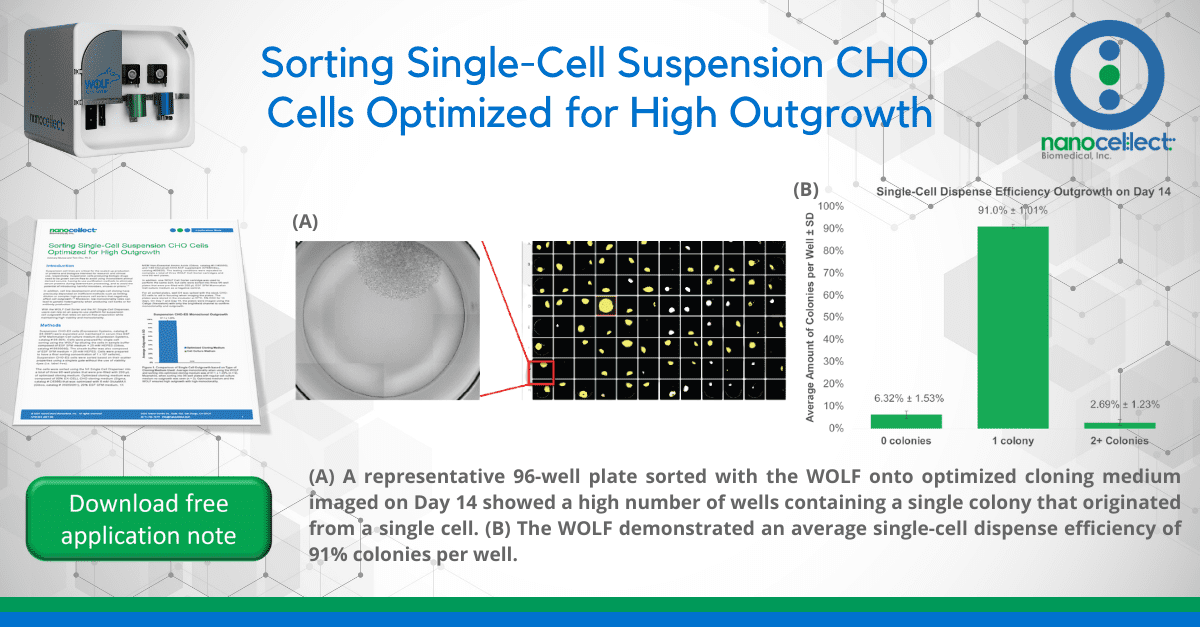
Figure 1. Comparison of Single Cell Outgrowth based on Type of Cloning Medium Used: Average monoclonality when using the WOLF and sorting into optimized cloning medium was of 97.1 ± 1.29% (n = 9). Meanwhile, when sorting into 96-well plates with regular cell culture medium no outgrowth was seen (n = 3). Optimized medium and the WOLF ensured high outgrowth with high monoclonality.
Results
Using optimized cloning medium and the WOLF we observed an enhanced outgrowth of the suspension CHO-ES cell line. The average monoclonal outgrowth analyzed on Day 14 was of 97.1 ± 1.29% when sorting into optimized medium compared to no outgrowth when sorting into regular cell medium (Figure 1). High outgrowth and efficient monoclonality can be observed when visualizing plates sorted with the WOLF into optimized cloning medium (Figure 2A). The WOLF resulted in an average total outgrowth of 93.4 ± 1.13% with 91.0 ± 1.01% of the wells containing a single colony with only one round of single-cell cloning (Figure 2B).
Other groups have reported limiting dilution for suspension CHO cells cloning with outgrowth efficiencies of less than 38.3 ± 10.4% after optimization.6 This further demonstrates that limiting dilution has low efficiency in producing monoclonality that is less than the predicted Poisson distribution.7 Furthermore, highly inefficient limiting dilution requires more cloning rounds to ensure monoclonal cell clusters.8
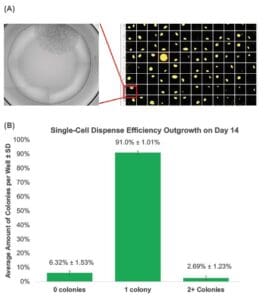
Figure 2. The WOLF Ensures High Monoclonality and Outgrowth: (A) A representative 96-well plate sorted with the WOLF onto optimized cloning medium imaged on Day 14 showed a high number of wells containing a single colony that originated from a single cell. Well D4 was spiked to facilitate plate imaging. Average total outgrowth when using the WOLF was of 93.4 ± 1.13% (B) The WOLF demonstrated an average single-cell dispense efficiency of 91.0 ± 1.01% with 6.32 ± 1.53% of the wells having no colonies and 2.69 ± 1.23% had two or more colonies per well.
Conclusion
The WOLF Cell Sorter is a critical tool for cell line development workflows that can be used for label-free or fluorescent activated single-cell sorting of suspension cell lines growing in serum free media. Additionally, the WOLF is capable of dispensing a single cell per well gently and with high accuracy to ensure monoclonality needed for cell line development and antibody production.
For more information, visit nanocellect.com
or email [email protected]
References
1. Miki, H., & Takagi, M. (2015). Design of serum-free medium for suspension culture of CHO cells on the basis of general commercial media. Cytotechnology, 67(4), 689–697. https://doi.org/10.1007/s10616-014-9778-0
2. Merten OW. Development of serum-free media for cell growth and production of viruses/viral vaccines–safety issues of animal products used in serum-free media. Dev Biol (Basel). 2002;111:233-57. PMID: 12678248.
3. Zhou, Y., Shaw, D., Lam, C., Tsukuda, J., Yim, M., Tang, D., Louie, S., Laird, M.W., Snedecor, B. and Misaghi, S. (2018), Beating the odds: The poisson distribution of all input cells during limiting dilution grossly underestimates whether a cell line is clonally-derived or not. Biotechnol Progress, 34:559-569.
https://doi.org/10.1002/btpr.2560
4. Andrä, I., Ulrich, H., Dürr, S., Soll, D., Henkel, L., Angerpointner, C., Ritter, J., Przibilla, S., Stadler, H., Effenberger, M., Busch, D.H. and Schiemann, M. (2020), An Evaluation of T-Cell Functionality After Flow Cytometry Sorting Revealed p38 MAPK Activation. Cytometry, 97: 171-183. https://doi.org/10.1002/
cyto.a.23964
5. Welch JT, Arden NS. Considering “clonality”: A regulatory perspective on the importance of the clonal derivation of mammalian cell banks in biopharmaceutical development. Biologicals. 2019 Nov;62:16-21. doi: 10.1016/j.biologicals.2019.09.006. Epub 2019 Oct 3. PMID: 31588011.
6. STEMCELL Technologies Inc. (2014). ClonaCell-CHO ACF Supplement: Robust Growth of CHO Cells at Low Density. STEMCELL Technologies. https://cdn.stemcell.com/media/files/brochure/BR29275-ClonaCell_CHO_ACF_Supplement_Low_Cell_Density.pdf?_
ga=2.179125732.1750382661.1616012776-1300914631.1601672139
7. Yeh CF, Lin CH, Chang HC, Tang CY, Lai PT, Hsu CH. A Microfluidic Single-Cell Cloning (SCC) Device for the Generation of Monoclonal Cells. Cells. 2020 Jun 18;9(6):1482. doi: 10.3390/cells9061482. PMID: 32570745; PMCID: PMC7349811.
8. Ye M, Wilhelm M, Gentschev I, Szalay A. A Modified Limiting Dilution Method for Monoclonal Stable Cell Line Selection Using a Real-Time Fluorescence Imaging System: A Practical Workflow and Advanced Applications. Methods and Protocols. 2021; 4(1):16. https://doi.org/10.3390/mps4010016
APN -024
