Sorting the Future of Cancer Research: How the WOLF® Cell Sorter Powers Tumor Microenvironment Studies
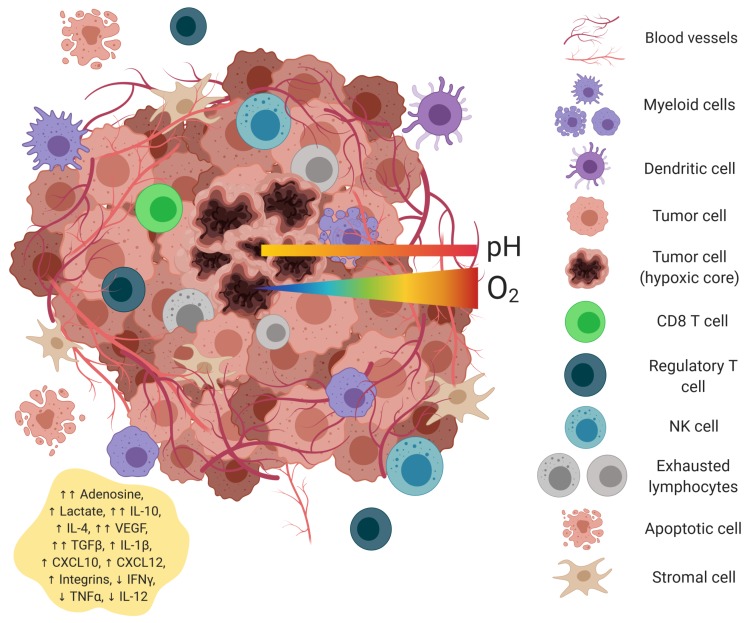
In the fight against cancer, we’ve long studied cancer cells and tumors themselves, but in recent years it’s become increasingly clear that was only part of the story. Tumors do not exist in isolation. Understanding the tumor microenvironment (TME), which is a dynamic ecosystem of immune cells, stromal cells, blood vessels, and extracellular matrix, shapes how tumors grow, spread, and respond to treatment.1
Figure 1. The tumor microenvironment is complex ecosystem of heterogenous cell types and environmental conditions. (Credit: Piñeiro Fernández, J. et al. 2019)
To uncover the complex cell-to-cell interactions at play, scientists increasingly turn to single-cell RNA sequencing (scRNA-seq) and other high-resolution methods. But there’s a catch: many cells within a tumor are fragile. Traditional high-pressure droplet sorters can damage them, introducing stress responses or killing them outright and compromising both data quality and downstream functional assays.
Why Cell Viability is Non-Negotiable in TME studies
For any study of the tumor microenvironment, the goal is to profile live cells in a way that maintains the inter-cell dynamics as it exists within a cancer patient’s body. Many TME-resident cells, like tumor-associated macrophages, cancer-associated fibroblasts, or exhausted T cells, are fragile and sensitive to shear stress. Preserving viability ensures accurate readouts in scRNA-seq and multiomics, functional immune assays (cytotoxicity, proliferation, cytokine release), and organoid or explant culture models.
How Gentle Cell Sorting is Furthering Oncology Research
Only with the integrity of the data can researchers elucidate new therapeutic strategies and targets. Researchers have therefore required a solution beyond traditional cell sorting methods that can damage cells, compromising viability and transcriptomic fidelity.
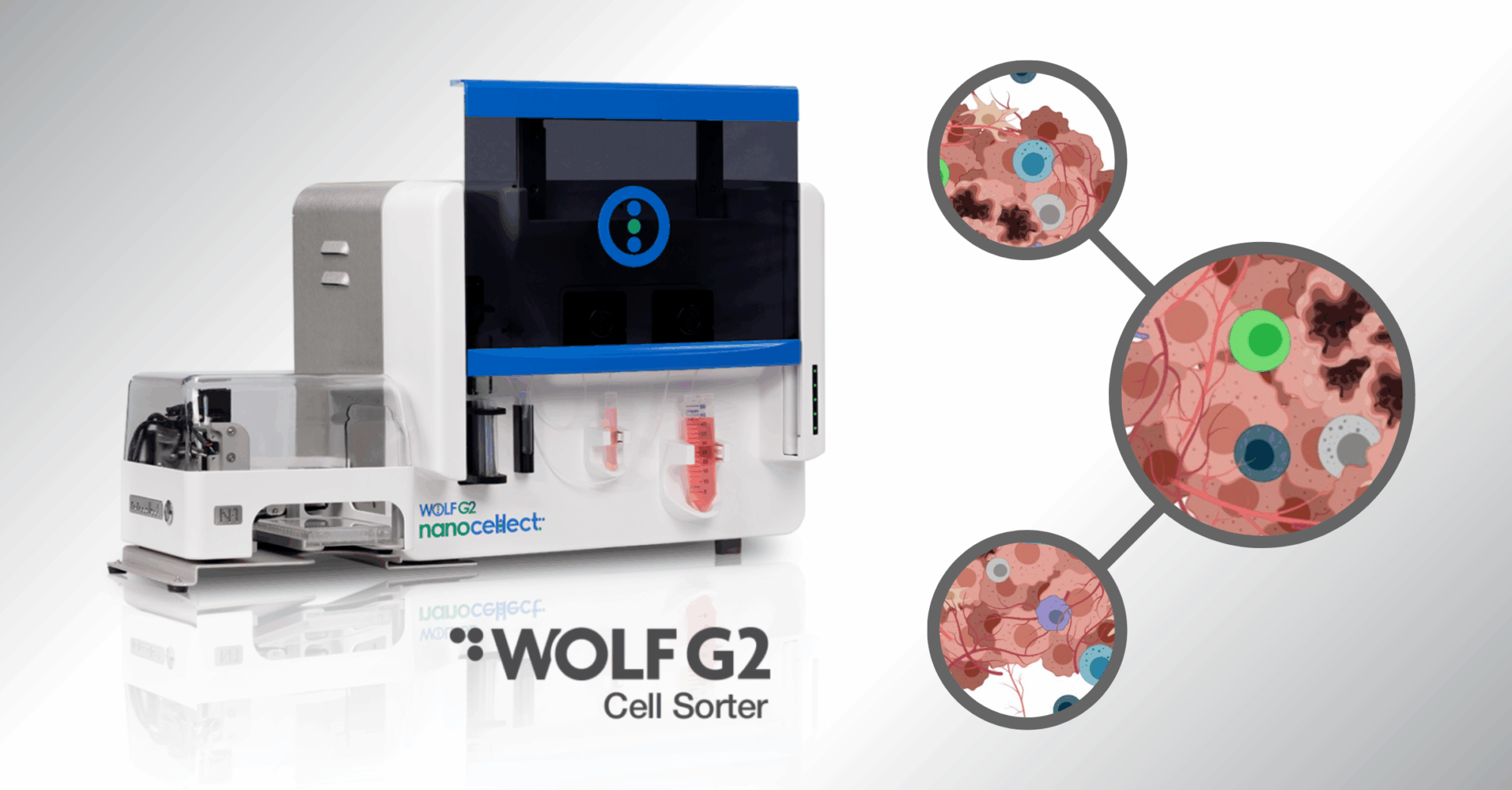
To overcome these challenges of TME research and viable cell isolation, there are increasing studies that have elected to use the WOLF cell sorting platform as their solution. Both the WOLF and WOLF G2 use microfluidic-based, low-pressure sorting to isolate specific cell populations without compromising viability. Key benefits for oncology researchers include:
• Preservation of Live Cells: Maintain RNA integrity and functional potential for downstream assays.
• Sterile, Closed-System Sorting: Reduce contamination risk, ideal for clinical or BSL-2 workflows.
• BSL-2 Cabinet Compatibility: Compact, benchtop-friendly design for safe handling of patient-derived material.
• Versatility Across Cell Types: Sort tumor cells, immune infiltrates, and stromal populations in one platform.
For cancer research, these advantages translate into higher-confidence data and the ability to run experiments that would be impossible with damaged or stressed cells.
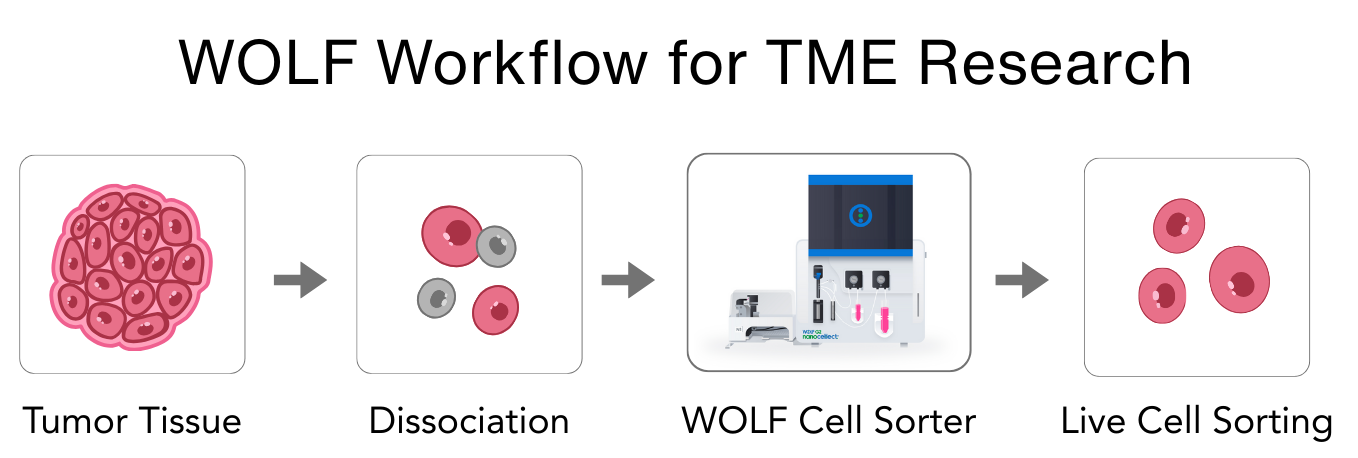
Figure 2. General workflow using the gentle cell sorting with fragile cell types isolated from tumors and their surrounding tissues.
How the WOLF has advanced our understanding of the tumor microenvironment
Case Study 1 – Mapping the Tumor-Immune Landscape
A 2021 Nature paper by researchers at Vanderbilt University studied cancer cell metabolism and compared it to tumor-infiltrating immune cells also present in the TME of a wide range of mouse tumor models.2 Across five different mouse tumor types, including colorectal, kidney, and breast cancers, they required isolated populations of live immune and cancer cells with high viability for scRNA-seq.
Using the WOLF Cell Sorter, they separated out immune cells, including delicate tumor-infiltrating immune cells and myeloid cells, as well as cancer cells. What they found was a detailed immune cell atlas that revealed how certain immune populations correlate with clinical outcomes. These insights were only possible by starting with healthy, intact cells. The team was able to identify distinct immune cell states and TME changes that correlate with patient outcomes, demonstrating the value of gentle, precise sorting for capturing the “true” biology of tumor-infiltrating immune cells.
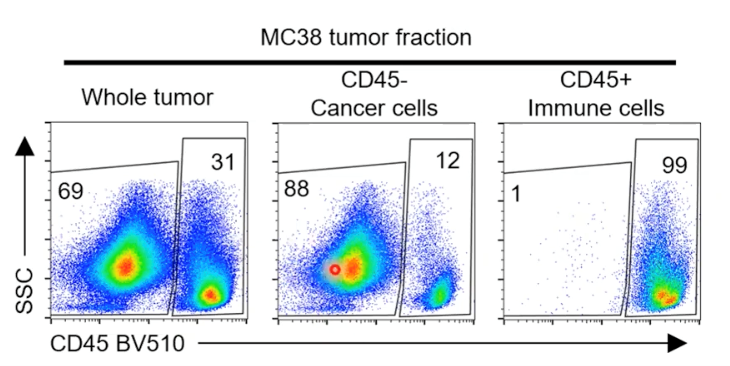
Figure 3. FACS by the WOLF Cell Sorter showing relatively pure fractions, allowing confidence in data and downstream study outcomes. (Credit: Reinfeld et al. 2021)
Using the WOLF, the team at Vanderbilt was able to further separate out different immune cell types and found that it is myeloid, not T cells, that have high glucose update. Understanding this metabolic heterogeneity in the TME has resulted in numerous subsequent studies, elucidating new therapeutic strategies based on metabolic heterogeneity within the TME.
Case Study 2 – Remodeling the TME with Combination Therapy
In this 2024 study, researchers tested whether combining PD-1 immune checkpoint blockade (a type of immunotherapy) with photodynamic therapy (a light-activated treatment) could reshape the tumor microenvironment in a mouse model of oral cancer. Using live, sorted cells, they discovered that the combination treatment not only slowed tumor growth but also:
• Reduced immunosuppressive regulatory T cells (which normally help tumors evade the immune system)
• Restored a subtype of fibroblasts linked to healthy tissue repair (Fib_Igfbp5)
• Shifted immune activity toward a more anti-tumor profile
The WOLF Cell Sorter was essential here as it gently isolated fragile immune and stromal cell populations from the tumors, keeping them alive and physiologically intact. This meant the sequencing data reflected the cells’ true biological state, rather than stress artifacts from harsh sorting.
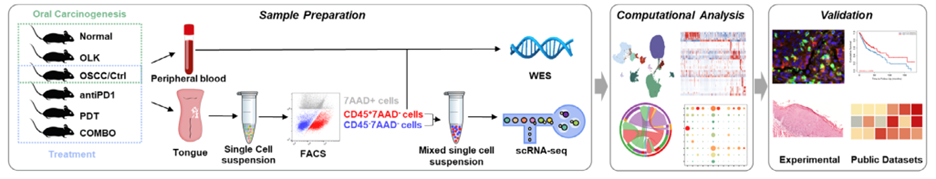
Figure 4: Overview of the whole experimental strategy: The dynamic features of microenvironment in oral carcinogenesis were dissociated and how these features change in response to treatment was tested. (Credit: Dong et al. 2024)
Case Study 3 – Stress, Immunity, and Breast Cancer Progression
In a 2025 Cell Death Discovery study, scientists investigated how chronic mental stress influences breast cancer progression in mice. Interestingly, the researchers found that stress didn’t just alter hormone levels—it actually rewired the tumor microenvironment. Stress exposure:
• Increased tumor-promoting immune cells (such as certain myeloid cells)
• Reduced tumor-fighting immune cells (like cytotoxic T cells and natural killer cells)
• Encouraged tumor cells to adopt a stem-like, treatment-resistant state
The WOLF Cell Sorter allowed the team to sort a wide variety of cell types, including immune cells, endothelial cells, and stromal cells, without harming them, enabling accurate single-cell profiling. This live-cell approach revealed subtle yet critical shifts in cell populations that help explain why psychological stress can worsen cancer outcomes.
Conclusion: Parsing Complex Interplays Between Cells to Understand Cancer Requires the Right Tools
Advancements in oncology hinge on the ability to analyze the TME in its native, living state. The WOLF and WOLF G2 Cell Sorters are more than instruments—they are enablers of discovery, offering gentle yet precise sorting that preserves cellular health. From metabolism mapping to therapy response and stress-induced tumor remodeling, these platforms empower researchers to unlock the TME’s secrets, one viable cell at a time.
References
-
- Piñeiro Fernández J, Luddy KA, Harmon C, O’Farrelly C. Hepatic Tumor Microenvironments and Effects on NK Cell Phenotype and Function. Int J Mol Sci. 2019 Aug 24;20(17):4131. (read article)
- Reinfeld, B.I. et al.Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021). (read article)
- Dong, Y. et al. Single-cell transcriptome dissecting the microenvironment remodeled by PD1 blockade combined with photodynamic therapy in a mouse model of oral carcinogenesis. MedComm 5, e636 (2024). (read article)
- Liu, P. et al.Single-cell RNA sequencing reveals the effects of mental stress on mouse mammary tumors and the tumor microenvironment. Cell Death Discov. 11, 328 (2025). (read article)