Induced pluripotent stem cells (iPSCs) are developmentally equivalent to embryonic stem cells (ESCs) in many aspects of their regenerative properties and proliferation. iPSCs hold advantages over ESCs partly because they lack the same ethical constraints posed by the scientific community. Exogenous factor-based reprogramming of somatic cells into a pluripotent embryonic stem cell-like state was described in Dr. Shinya Yamanaka’s seminal work in 2006.1 Yamanaka’s discovery enables current iPSC technology; furthermore, iPSC technology enables ethical derivation of instrumental tools vital to research spanning fundamental biology, regenerative medicine, drug discovery and, more recently, food sciences.
The culture, handling, and sorting of induced pluripotent stem cells require special care for reliable results. Culturing iPSCs is a time consuming and laborious process with challenges that require a substantial commitment of energy and resources. iPSC technology has increased the availability of cells to study many applications that are otherwise difficult or impossible, such as neuroscience and predictive disease modeling.2 Maintaining the naïve state and pluripotency (ability to differentiate into three primary germ layers) is reliant on regulating variables such as nutrient composition, temperature, and other developmental cues. Differentiating cultures are heterogeneous, and although maintaining a homogeneous stem cell culture is possible, researchers often need other tools to succeed. The WOLF cell sorter is advantageous to the success of gently sorting homogeneous stem cells and eliminating unwanted cells. In addition to the WOLF cell sorter, the WOLF G2 can also facilitate these gentle sorts by increasing the color capabilities with the addition of a second laser. The 488 nm and 637 nm lasers were leveraged to obtain approximately 93% percent purity and > 99% viability using the methods as outlined below. In addition, NanoCellect’s microfluidic sorting technology together with defined and animal-free growth medium that enhances retention of naivety and pluripotency, enables researchers to generate consistent high-quality results.3,4
Fluorochrome-conjugated antibodies directed to surface antigens can verify whether stem cells have maintained or lost indicators of pluripotency. Here we demonstrate that the cell surface markers SSEA-4 (Stage-specific Embryonic Antigen-4) and TRA-1-60-R (Tumor-related Antigen-1-60 [R]), expressed by naïve ESCs and iPSCs, are robust surface markers for gentle microfluidic enrichment of hiPSCs on the WOLF G2. Expression of both SSEA-4 and TRA-1-60-R is downregulated following cell differentiation.5
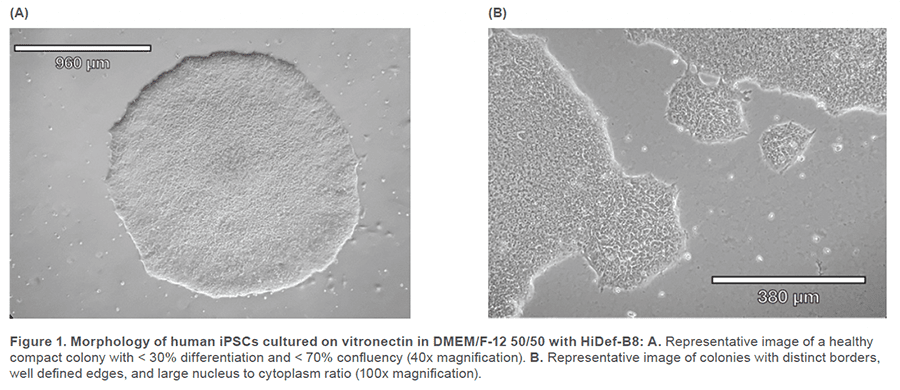
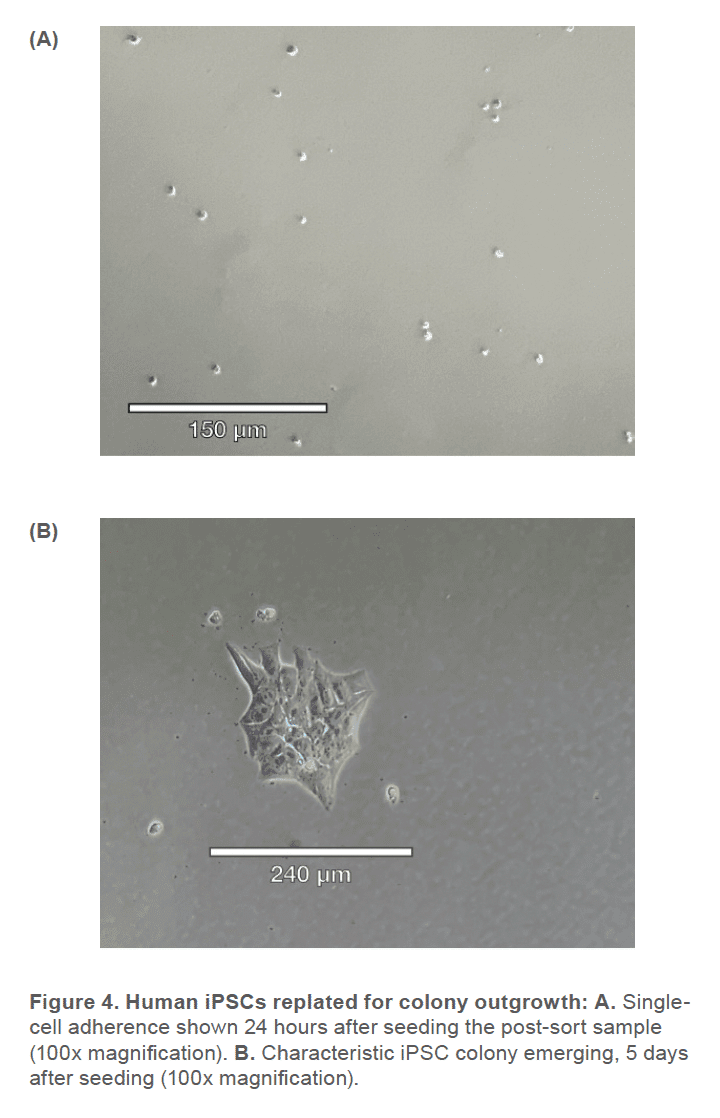
Human iPSCs reprogrammed from fi broblasts were cultured in DMEM/F-12 50/50 1X (Corning™ #10-092-CM) supplemented with HiDef B8 500X (Defined Bioscience #LSS-201) according to manufacturer’s instructions. Non-TC treated 6-well plates (CELLTREAT #229506) were treated with vitronectin (Gibco™ #A14700), a recombinant human protein that provides a defined surface for feeder-free culture. Samples were maintained with a visual assessment of < 30% differentiation per well. Cells were passaged in aggregates ranging from 50-100 μm, using the enzyme-free Gentle Cell Dissociation Reagent (GCDR, STEMCELL Technologies #100-0485). Manual negative removal of differentiated cells and positive harvesting of healthy cells was also performed by scoring colonies into a grid-like pattern and using a sterile 10 μL pipette tip to manipulate the grid pieces. Healthy colonies were determined by examining the morphology under phase microscopy for colony compactness, distinct borders, well defined edges, and large nucleus-to-cytoplasm ratio (Figure 1). Cell passages occurred when colonies were at least 600 μm in size, before colonies merged with each other (confluency < 70%) and plated with split-ratios ranging from 1:3 – 1:10. A single-cell suspension was obtained using Accutase® (Innovative Cell Technologies, Inc. #AT104) at 1 mL per well for 6-8 minutes, at 37°C. Cells were quenched with media, aggregates were lifted with a sterile cell lifter, the cell suspension was centrifuged at 200 x g for 3 minutes and resuspended in sheath buffer at a concentration of ~ 3.0 x 105 cells/mL. For optimal performance, complete medium was supplemented with 10% Accutase and used as sheath and sample buffer. Unstained cells, nonviable Sytox Green cells, SSEA-4 APC-conjugated antibody (R&D Systems #FAB1435A-025) stained cells and TRA-1-60-R PE/Cyanine7-conjugated antibody (BioLegend #330619) stained cells were used as controls. The SSEA-4 and TRA-1-60-R doublepositive post-sort sample was seeded into a 6-well plate with a final vitronectin coating concentration of 0.5 μg/cm2 and a low seeding density of ~ 5 x 104 cells/well. Single-cell adherence images were taken on Day 1 and emerging colony images were taken on Day 5 post-cell-sort (Figure 3).
Sorting of SSEA-4 and TRA-1-60-R positive iPSCs
Prior to sorting, approximately 69% of the cells were positive for SSEA-4 and TRA-1-60-R (Figure 2A) and roughly 6% of the cell sample included dead cells (Figure 3A). After using the gate SSEA4+TRA160+ (top right quadrant) to sort for cells that were positive for both surface markers, the double-positive cells were increased to 93% (Figure 2B). Greater than 99% SYTOX™ Green Ready Flow™ Reagent dead cell stain (Figure 3B), reflecting a high viability following microfluidic sorting.
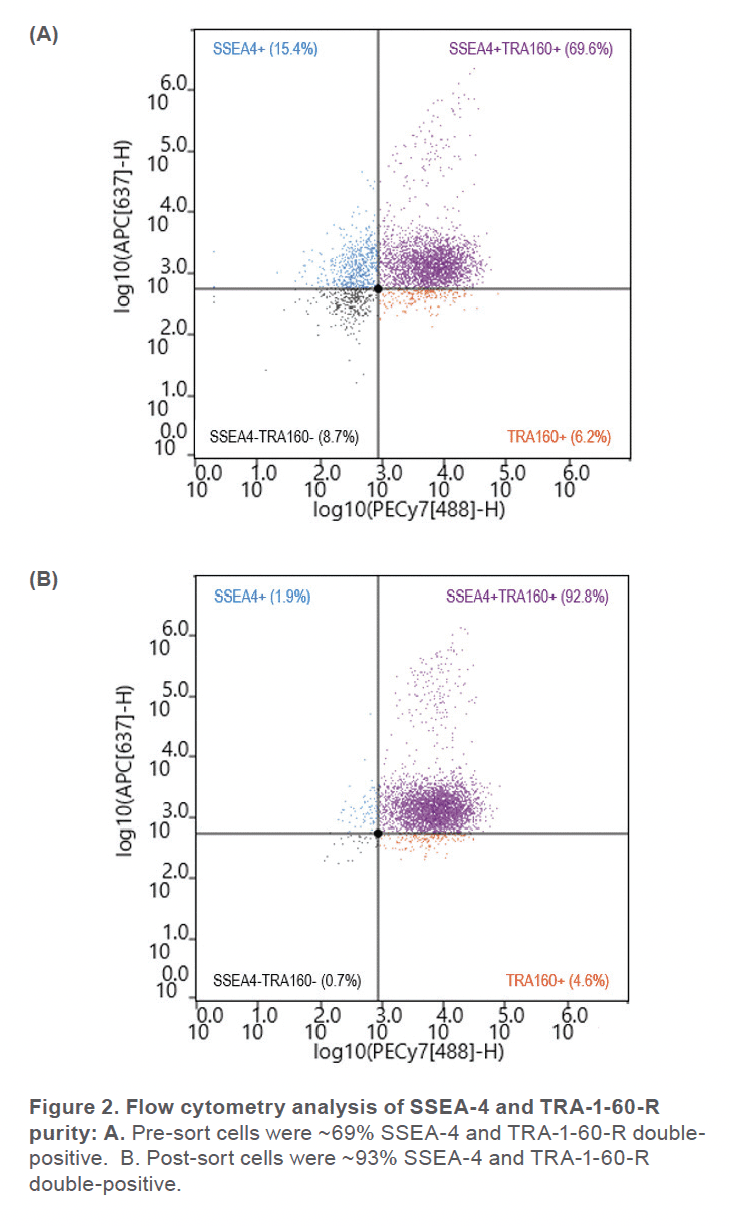
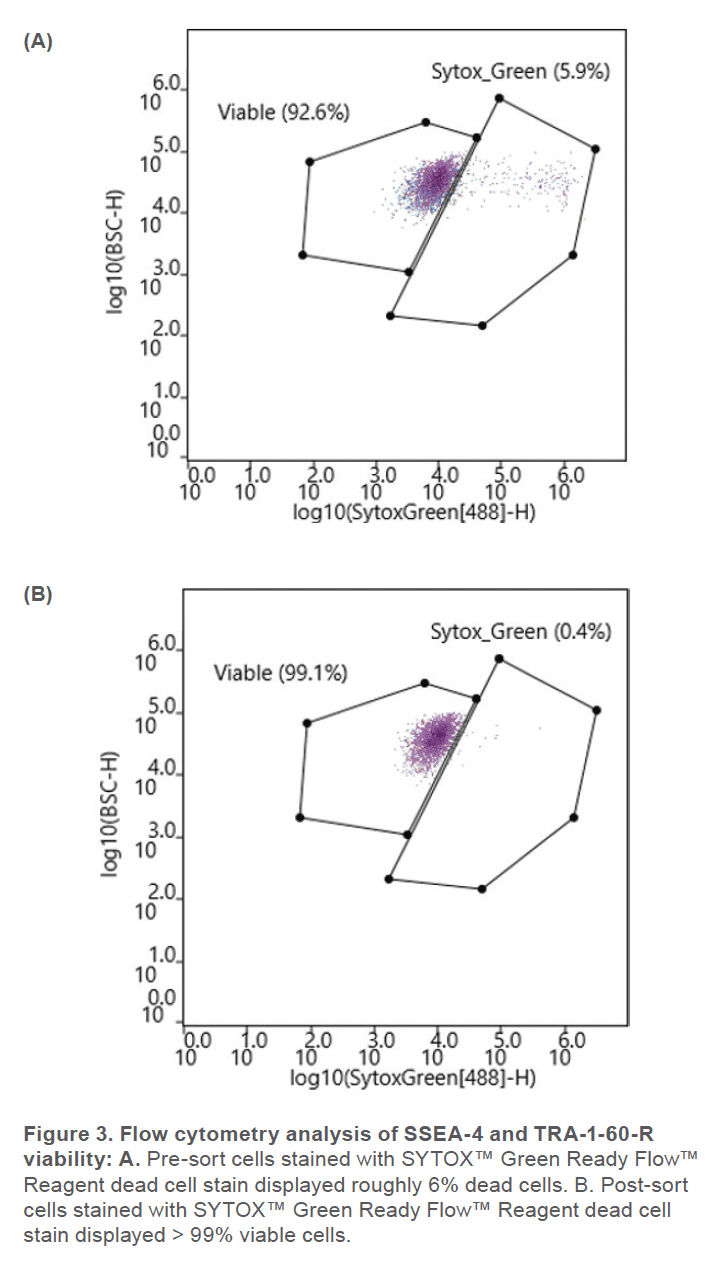
The WOLF G2 accurately identified and enriched hiPSCs that were labeled with two surface markers, SSEA-4 and TRA-1-60-R, widely used to label undifferentiated stem cells. The WOLF G2’s ability to identify and sort these cells is imperative for research and development when naïve stem cell populations are needed. Considering that this panel contained fluorophores that lye in the excitation range of the 488 nm and 637 nm lasers, a two-laser system such as the WOLF G2 (488, 637) was therefore vital to successfully performing this experiment. Using these surface markers proved successful by starting with a 69.6% double-positive sample and purifying to 92.8%. The indicated demonstrates that this microfluidic cell sorter was able to purify the initial sample by successfully detecting cells that were actively expressing both surface markers. This level of purification and > 99% viability demonstrates that the WOLF G2 is helpful in stem cell applications, such as neuroscience and disease modeling. Overall, the gentle sorting and positive identification of such a demanding and delicate cell type has demonstrated the high utility of the WOLF G2.
For more information, visit nanocellect.com or email [email protected]
APN -033





