WOLF Cell Sorter: T Cell Isolation and Functional Analysis
Introduction
T cells play a pivotal role in the immune system by helping our bodies fight antigens produced by viruses, bacteria and cancer cells. T cells are of interest and importance for immunotherapy and infectious diseases. Isolated T cells can be used in several downstream applications such T cell culture, T cell activation and expansion, genetic engineering / CAR-T, and other molecular studies. Specifically, adoptive T cell therapies have been impactful in the development of cancer immunotherapies for B-cell lymphoma and metastatic melanoma. In addition, isolation of antigen specific T cells has been instrumental in the development of vaccines. These studies require the ability to effectively and gently sort T cells for downstream genetic modification or functional studies. The WOLF Cell Sorter is a gentle microfluidic cell sorter capable of sorting fragile cell types with the least mechanical stress. Here, we demonstrate the capability of the WOLF to gently sort viable T cells for downstream applications.
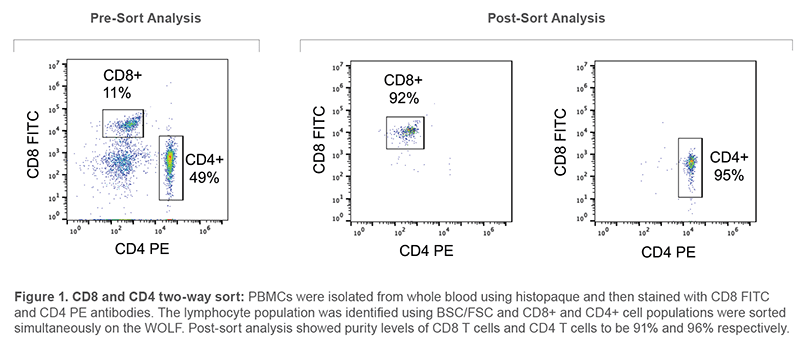
CD4+ and CD8+ T Cell Two-way Sorting When trying to isolate more than one cell type from a heterogenous sample, the WOLF offers the convenience of being able to sort two different types of T cells at the same time. To demonstrate this ability, human PBMCs were obtained from a histopaque gradient from whole blood (SD Blood Bank). Cells were stained with CD8-FITC (BioLegend, #344703) and CD4-PE (BioLegend, #317409) antibodies. 500,000 cells were concentrated to 90 μL of staining buffer with 5 μL of each antibody. Cells were washed and diluted to 200 cells/μL before sorting. A compensation matrix was used to mitigate spectra overlap between the FITC and PE channels using the WOLFViewer software. The lymphocyte population was first gated and then the CD4+ and CD8+ populations were then sorted based on the unstained negative control population (Figure 1). Bulk sorting was used to sort CD8+ cells into channel A and CD4+ cells into channel C. The BD Accuri™ C6 was used to analyze the purity of the CD4 and CD8 populations post-sort (Figure 1).
Results
After sorting CD8+ and CD4+ cells at the same time, purity was 92% and 95%, respectively. These results show that the WOLF is able to sort two different T cell types simultaneously and at high accuracy.
T Cells Maintain Viability After WOLF Sorting
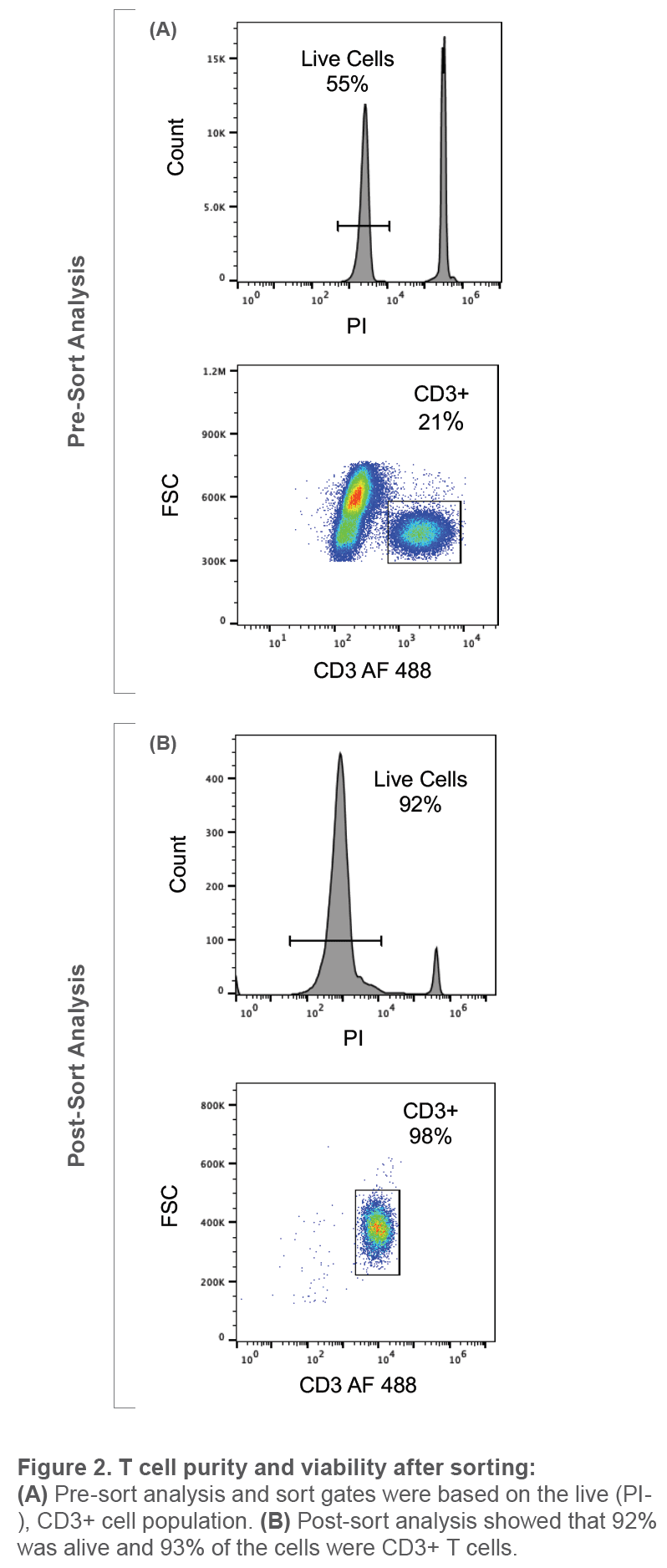
Maintaining healthy cells after sorting is critical for downstream applications such as cell culture and next generation sequencing. Conventional cell sorters can cause a significant amount of cell death, limiting the number of downstream applications that can be used after sorting. In order to determine the viability of T cells after sorting, PBMCs were first isolated with histopaque and then stained with CD3 AlexaFluor 488 (Biolegend, #300415). These cells were then diluted to 250 events/μL and stained with propidium iodide (PI) to determine cell viability and sort viable CD3+ cells. Cells were then analyzed and sorted on the WOLF Cell Sorter using DPBS + 2% FBS as sheath. T cell sorting gates were based on the PI negative (live cells), CD3+ population (Figure 2A). 10,000 live CD3+ cells were then sorted into the C channel on the microfluidic cartridge. Sorted cells were then stained again with PI to assess viability after being sorted on the WOLF Cell Sorter. The BD Accuri™ C6 was used to analyze the purity and viability of the CD3+ population after sorting.
Results
Pre-sort analysis on the WOLF showed that 55% of the PBMC cell population was alive and 21% were CD3+. After sorting the CD3+ live cells, post- sort analysis revealed that 92% of the population was alive and 98% of the cell population was CD3+. These results show that the WOLF is able to successfully deplete the dead cell population while resulting in little to no cell death. In conclusion, this experiment demonstrates the ability of the WOLF to sort fragile cell types and maintain high viability. When using a viability dye to eliminate dead cells on the WOLF, T cells will still be viable after sorting, ensuring high quality results for your downstream experiments.
Sorted T Cells Maintain Function After Sorting
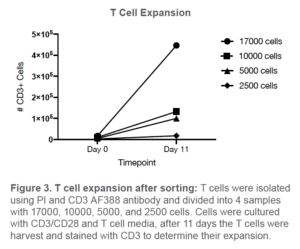
To further confirm that T cells are not only viable, but also healthy enough for downstream functional assays, we performed a T cell expansion assay. CD3+ viable cells (98% purity) were bulk sorted into 4 samples of 17,000, 10,000, 5,000 and 2,500 cells with STEMCELL™ Technologies ImmunoCult™-XF T cell Expansion Media (#10981) supplemented with
IL-2 (10 ng/mL). Each sample was then stimulated with ImmunoCult™ Human CD3/CD28 T Cell Activator (#10971) and fresh media was added to each sample every 2-3 days. After 11 days, all of the samples were harvested and stained with PI and CD3 AF488. All samples were then analyzed on the BD Accuri TM C6.
Results
All four of the T cell samples demonstrated expansion capacity and maintained viability ranging from 81-85%. The highest rate of growth was observed from the sample that started with 17,000 sorted CD3+ T cells (Figure 3). Lower rates of expansion were observed from the lower starting cell concentrations; however, T cells were still present and viable which shows that the WOLF has little adverse effect on T cells.
In conclusion, these results show that the WOLF can serve as a robust T cell sorter for T cell functional assays. Experiments such as preclinical adoptive cell therapies of antitumor or antiviral T cells, genomic studies, and other T cell functional assays can be successful using the WOLF. Together, these results show that the WOLF is a compatible upstream platform
for T cell assays.
APN-016