The WOLF G2 Cell Sorter is a microfluidic cell sorter the uses sterile, disposable sorting cartridges. In addition, the WOLF G2 has 2 lasers than can allow up to 9 fluorescent colors on the 488nm/405nm configuration. This empowers users to characterize multiple different cell types within a population. This is especially critical for scientists who are looking to
characterize an immune response following infection or vaccination. Here, we demonstrate a 9-color leukocyte panel using the WOLF G2 488nm/405nm configuration.
BioLegend’s Veri-Cells™ (#426004) were used for this panel design. After reconstituting the leukocytes, the cells were treated with Human TruStain FcX™ (BioLegend, #422301) to reduce nonspecific binding. The cells were then stained with anti-human BioLegend antibodies: CD45 Alexa Fluor® 488 (#368535), CD3 Brilliant Violet 711™(BV711) (#317327), CD4 Brilliant Violet 605™ (BV506) (#300555), CD8 Brilliant Violet 421™ (BV421) (#344747), CD19 PE/Dazzle™ 594 (#302252), CD66b Biotin (#305120), CD14 Brilliant Violet 510™ (#301841), CD56 PE/Cyanine5 (PE-Cy5) (#318308) and CD45RA PE (#304107). The cells were then stained with a secondary antibody Qdot™ 565 Strepavidin (ThermoFisher, #Q10133MP) and then finally diluted to 3 x 105 cells/mL before analysis. Compensation beads (Slingshot, #SSB-05A) were used to apply compensation to the sample. Gain settings were as followed: FL1- 400 mV, FL2- 400 mV, FL3- 400 mV, FL4- 480 mV and FL5- 550 mV. Gate placement was confirmed through backgating and fluorescence minus one (FMO) controls.
All nine fluorescent antibodies corresponding to the specified cell types were identified. The leukocyte population was first identified through the BSC/FSC plot (Figure 1A) then singlets were identified by the FSC-W/FSC-H plot (Figure 1B). Leukocytes were further isolated from debris using CD45 (Figure 1C). From the CD45+ parent gate, Granulocytes
(CD66b+) and T cells (CD3+) were identified through a CD66b Qdot 565/CD3 BV11 scatter plot (Figure 1D). A gate was then drawn around the CD66b-CD3- cells and within that cell population, monocytes (CD14+), B cells (CD19+) (Figure 1E) and NK cells (CD56+) (Figure 1F) were identified. CD4 and CD8 T cells were recognized from the CD3+ parent gate as CD4 BV711+ and CD8 BV421+ (Figure 1G). Lastly, independently, from both the CD4 and CD8 parent gate, naïve (CD45RA+) and memory (CD45RA-) CD4 or CD8 T cells were discovered (Figure 1H-I).
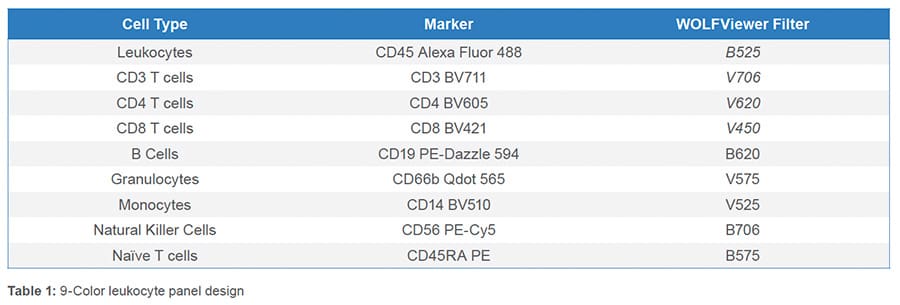
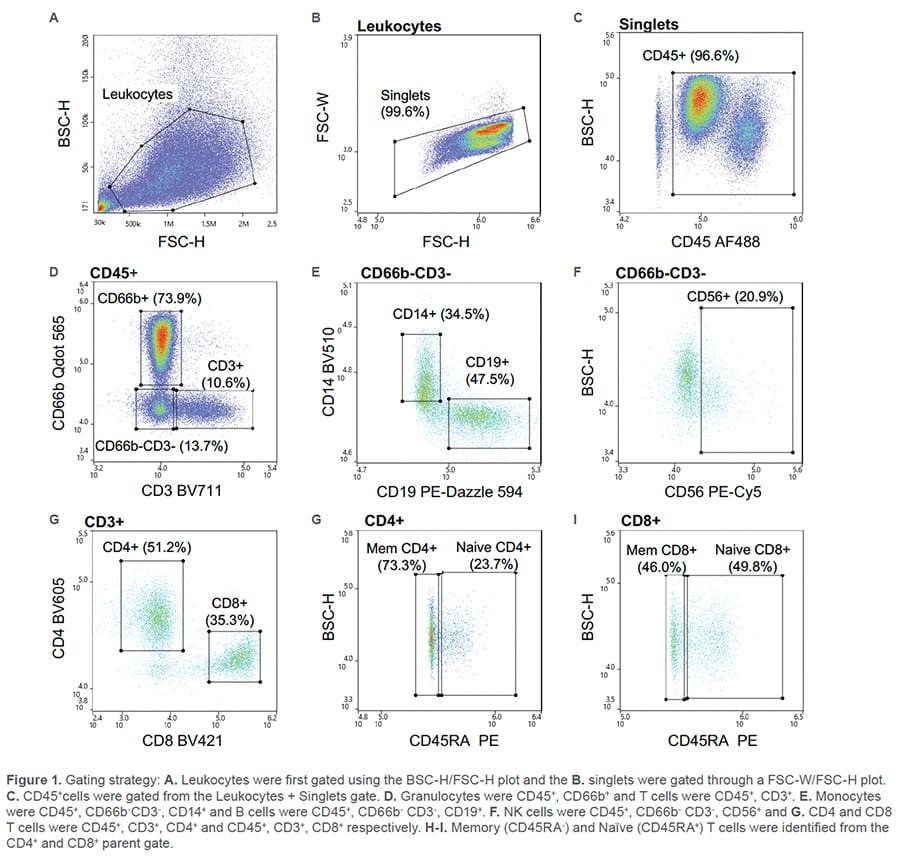
Nine fluorescent antibodies were successfully used to identify multiple cell types within the leukocyte population. This data demonstrates the broad capabilities of the WOLF G2 488nm/405nm configuration.
For more information, visit nanocellect.com or email [email protected]
TCN – 009





