5-Color Panel Design on the WOLF G2 Single Laser (SL) Cell Sorter
Introduction
The WOLF G2® Single Laser (SL) Cell Sorter is NanoCellect’s solution to support customer’s desire to maximize usage with a single laser system. The WOLF G2 SL has the same design as the WOLF G2 which means that users can now place the single laser unit forward-facing in a tissue culture hood. In addition, the WOLF G2 SL sorter can be upgraded to a 2-laser system at a later date. Lastly, it uses the same innovative sorting technology with more sorting parameters. Instead of 3 fluorescent channels, the WOLF G2 SL has 5 fluorescent channels along with forward scatter and back scatter. This means that users can use two additional fluorescent markers in their panel design, as compared to the WOLF G2. This technical note demonstrates the broader analysis capabilities of the WOLF G2 SL with a 5-color panel.
Method
BioLegend® Veri-Cells™ Leukocytes (#426003) were used for this panel design. After reconstituting the leukocytes in the Buffer A Plus supplied in the kit, the cells were treated with Human TruStain FcX™ (BioLegend, #422301) to reduce nonspecific binding. The cells were then stained with anti-human BioLegend antibodies: CD66b PE-Cy7 (#305115), CD19 PE-Dazzle™ 594 (#302252), CD3 PE-Cy5 (#300410), CD4 PE (#300508) and CD8 FITC (#301006) (Table 1). The sample was then diluted to 2 x 105 cells/mL before analysis and sorting. Compensation beads (Slingshot, #SSB-05A) were used to apply compensation to the sample. CD4+ and CD8+ T cells were sorted simultaneously. Post-sort analysis was also done on the WOLF G2-SL.
Results
The leukocyte population was first identified through the BSC/ FSC plot (Figure 1A), then singlets were identified by the FSC-W/FSC-H plot (Figure 1B). PBMCs and granulocytes were separated using a BSC/CD66b PE-Cy7 plot (Figure 1C). T cells (CD3+) and B cells (CD19+) were then identified through PBMC gate (Figure 1D) and CD4+ and CD8+ T cells Were identified from the CD3+ parent gate (Figure 1E). Pre-sort CD4+ T cells made up 5.9% of the leukocyte population and CD8+ T cells made up 5.2% of the leukocyte population. Post- sort purity of CD4+ and CD8+ T cells were 91.9% and 92.5%, respectively (Figure 2).
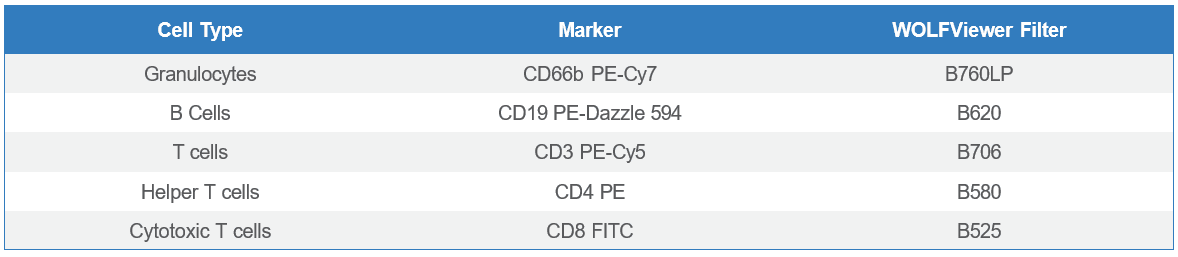
Table 1: 5-Color leukocyte panel design
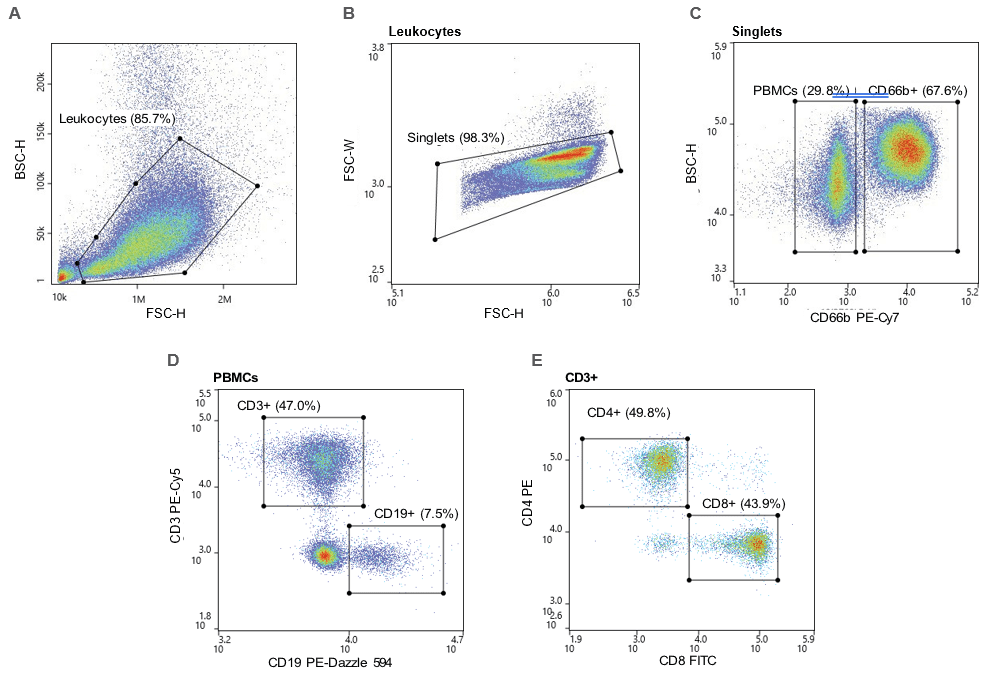
Figure 1. Gating strategy: A. Leukocytes were first gated using the BSC-H/FSC-H plot and the B. singlets were gated through an FSC-W/FSC-H plot. C. PBMCs were CD66b- and granulocytes were CD66b+ D. T cells were CD66b-CD3+ and B cells were CD66b-CD19+. E. CD4+ and CD8+ T cells were CD66-CD3+CD4+ and CD66-CD3+CD8+, respectively.
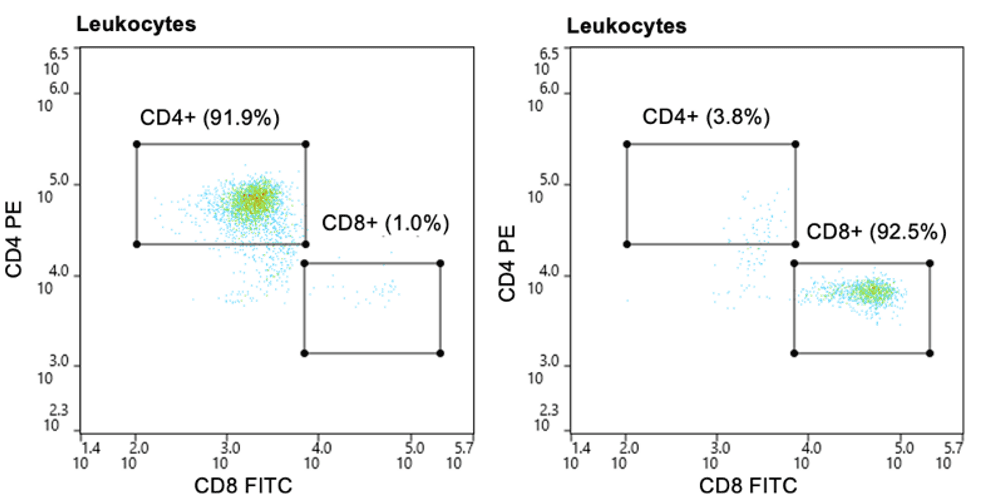
Figure 2. Post-sort purity of CD4+ and CD8+ T cells: CD4+ and CD8+ T cells were purified to approximately 92% of the leukocyte population post-sort.
Conclusion
The WOLF G2-SL is a 488nm single laser microfluidic cell sorter that can sort cells with up to 7 parameters: back scatter, forward scatter, and 5 fluorescent channels. This technical note shows that all 5 fluorescent antibodies corresponding to the specified cell types were clearly identified on the WOLF G2 SL. In addition, this data demonstrates that the WOLF G2 SL has the capability to sort target populations with the expected high purity.
For more information, visit nanocellect.com and abterrabio.com or email [email protected]
TCN-010
