NanoCellect is developing the VERLO Image Guided Sorter to allow researchers to see and sort their cells. The name comes from Spanish, ‘ver lo’ that roughly translates to ‘see it’.
We are anticipating the VERLO will offer several key advantages over conventional cell sorters.
Conventional flow cytometry and sorting collect a single point of data per detector from each particle. This is essentially a one-pixel picture for each channel of fluorescence and scatter. Approximately 20 years ago, imaging cytometry was commercialized by Amnis. While sorting has been recognized as a logical extension of that work, imaging cell sorters have not been available. NanoCellect devices will allow Spatial CytometryTM and Spatial SortingTM by designing and building Spatial CytometersTM and Spatial SortersTM. The VERLO will be a both a Spatial CytometerTM and a Spatial SorterTM.
1.1. Image-Guided Cytometry – definitions, principles and uses
Imaging cytometry is the ability to collect spatial information, or images, from thousands or millions of cells or particles suspended in flow. This can be used to identify and sort cells that display, or have been rescued from, a specific phenotype—such as the accumulation of a protein due to abnormal processing. Sorted cells can be used for subsequent clonal expansion in culture or analyzed by ‘omics techniques of genomics, transcriptomics or proteomics. Image guided sorting can also be used for the selection and sorting of immune cells that are interacting with a counterpart cell to carry out the cellular activities of immune-oncology or infectious disease.
1.2. Analysis and Sorting
Imaging flow cytometry requires very rapid (sub-millisecond) acquisition of the images to support the high throughput expected in flow cytometry. These collected images can be analyzed by a number of well-established metrics, such as area, ratio of height to width, identification of organelles, and the location of proteins or material taken up by cells. In the case of cell sorting, these metrics can be used to create gates for positive or negative selection and cells can be sorted based on these gates. While analysis of images from thousands of cells is computationally intensive for analysis-only imaging cytometry, there is no need for real-time analysis. In the case of image-guided cell sorting, however, image features, gates, and sorting decisions need to be made in sub-millisecond time scales. This requires very fast computation that has not been previously affordable for researchers.
In addition to using conventional image features, we have developed a method to use Artificial Intelligence (AI) to help identify and sort cells—even when unlabeled—with real-time AI guided sorting.
2.1. Principal components of a image guided cell sorting
A flow cytometer is a complex instrument composed of three main systems: fluidics, optics, and electronics. When the three systems work in conjunction with one another, the user can acquire a great deal of information about a given cell or population.
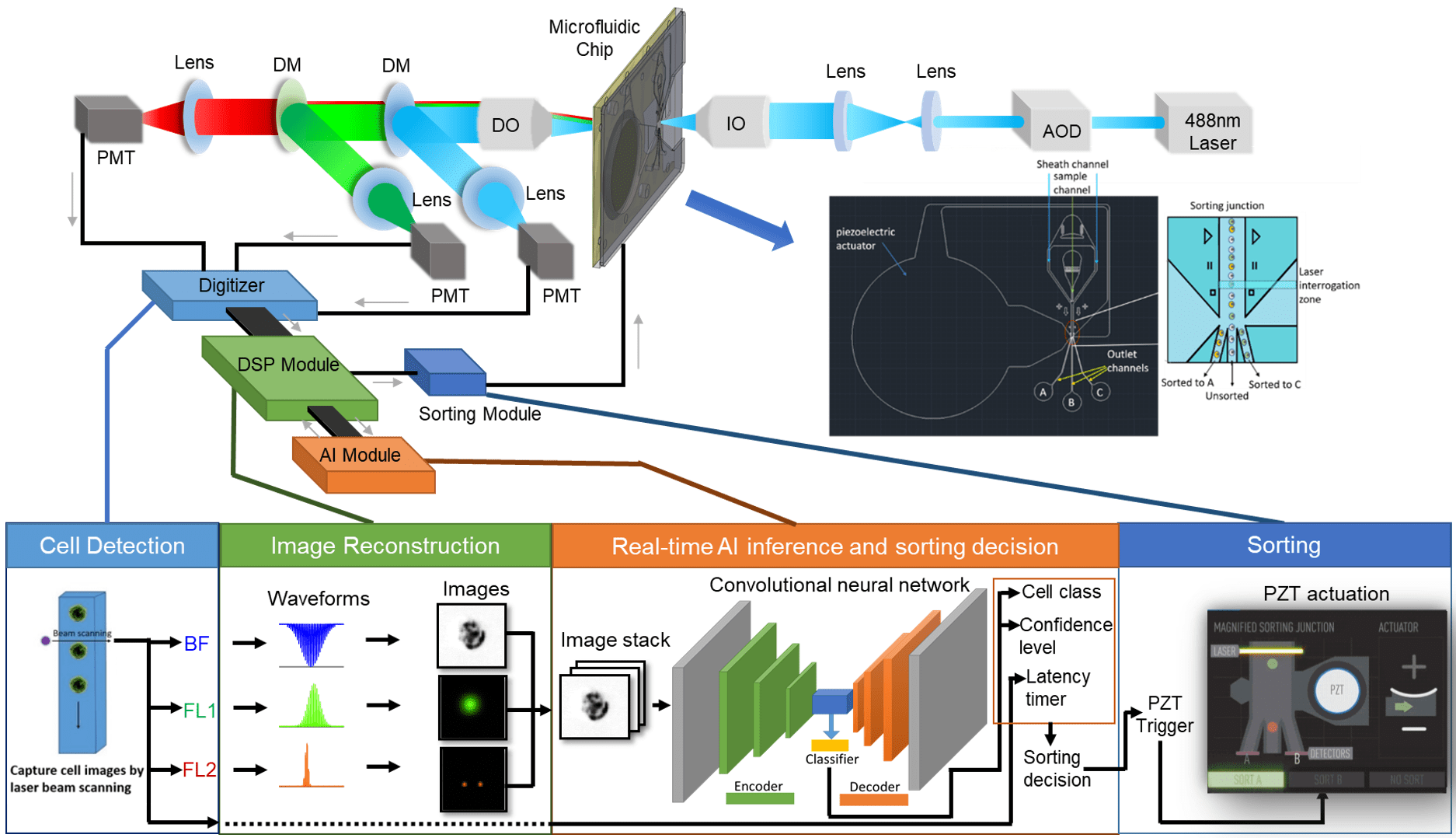
Each of the three systems serves an integral purpose leading to the acquisition of data.
The fluidics system transports the cell sample from its tube, through the pump system and flow cell, and to an outlet port for collection.
The optics system is responsible for illuminating the sample, as well as collecting and directing the scattered light and emitted fluorescent light to the detection system.
The electronics system is responsible for digitizing and further processing the signals obtained from the detectors for subsequent analysis.
In flow sorters, the incorporation of a sorting mechanism enables the capture of a specific subset of cells from a heterogeneous population.
2.2. Fluidics System
NanoCellect has near 20 years of experience in the design, manufacture, and support of microfluidic cell sorters. The VERLO Imaging Cell Sorter will take advantage of these technologies to image and sort high-viability, high RNA-integrity cells in a sterile, gentle, zero-carryover cartridge for bulk and plate (96- and 384-well) sorting.
2.3. Optics System
The Verlo Imaging Cell Sorter will be equipped with more than 1 laser to generate several channels of image data from both the label-free and fluorescent regimes. Unlike a conventional flow cytometer or sorter that creates one pixel per particle, our imaging system is capable of collecting 1,600 pixels of information per particle with best-in-class sensitivity, dynamic range and signal-to-noise characteristics.
2.4. Electronics
In order to collect data from so much image data, analyze it in real time, and make a sorting decision, we have to use the latest in FPGA, signal processing, and computer technologies. Within the VERLO Imaging Cell Sorter, there are several detectors that are constantly collecting data for label-free and fluorescent analysis. When a cell enters the laser beam, there will be a measurable increase, pixel-by-pixel, in signal over the baseline.
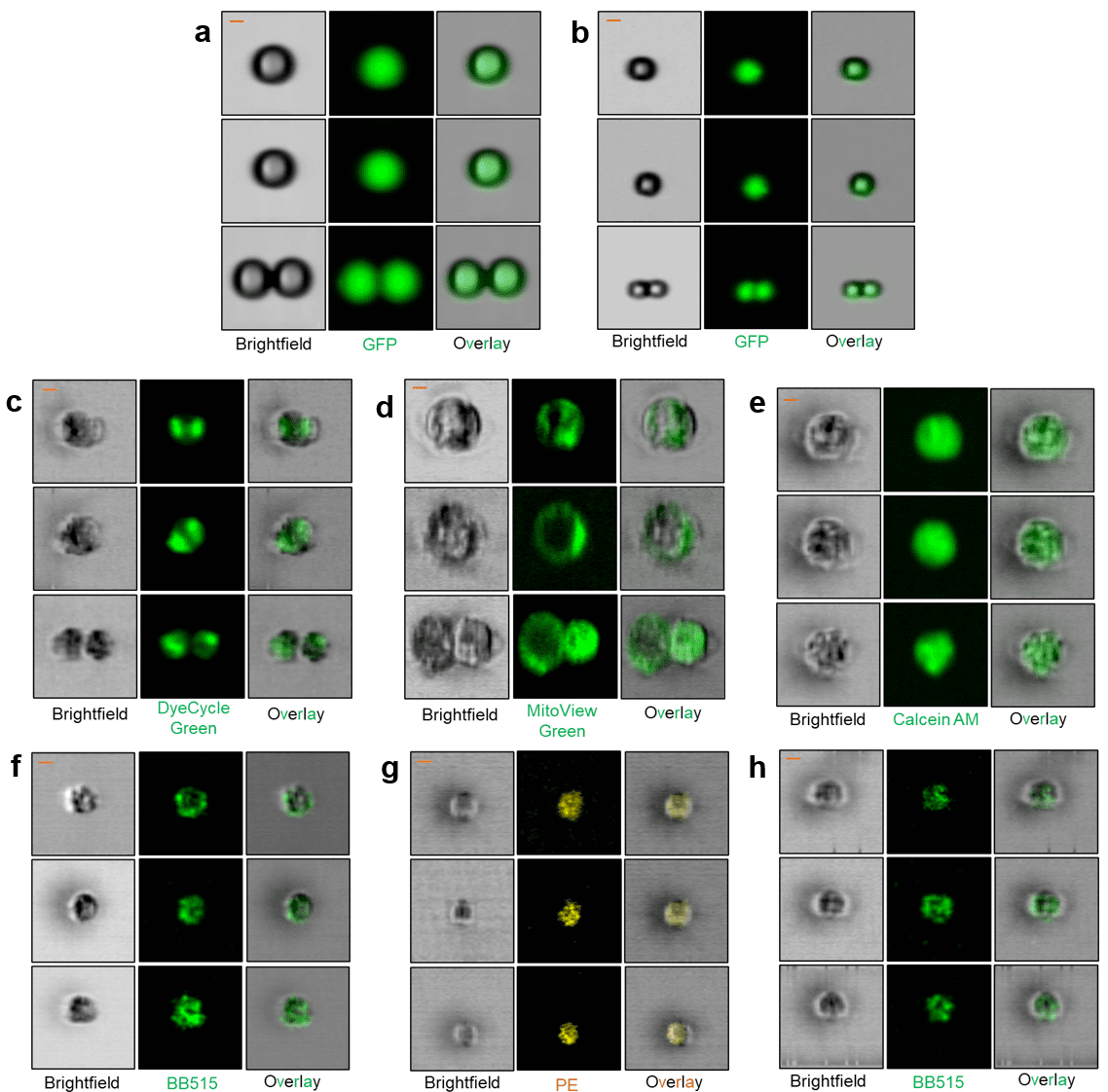
The microfluidic WOLF, WOLF G2, and VERLO Image Guided Cell Sorters use the same sterile, disposable, gentle microfluidic cartridges to assure high viability and a best-in-class biohazard profile to protect users. The ability to add the use of images to select and sort cells will allow “seeing is believing” to definitively select or avoid doublets, select cells with protein localization in the nucleus or other organelles, and do functional genomics to induce or rescue phenotypes of interest—such as the accumulation of pathogenic or misfolded proteins implicated in neurodegenerative disease.
NanoCellect, in close collaboration with co-founder and Chairman of our Scientific Advisory Board, Professor Yuhwa Lo, PhD, has been awarded over $13 million in National Institutes of Health (NIH) Small Business Innovative Research (SBIR) Awards. NanoCellect has received the Small Business Administration’s Tibbetts Award in recognition of our ability to invent, manufacture, sell, and support our systems with biomedical researchers globally.