Gentle Sorting of T Cell Subsets with the Dual-laser WOLF G2
Download PDF
Introduction
High-parameter T cell subset isolation is desirable for many research applications. It reveals how naïve, central memory, and effector memory T cells affect the efficacy and longevity of CAR T-cell therapy.1 It elucidates immune response mechanisms against diseases like COVID-19.2 And it uncovers antigen-specific T cell function3 for use in vaccine development and cancer immunotherapy.
Isolating specific T cell subsets like these can be difficult since it requires numerous selection parameters to tag all appropriate biomarkers and generally requires the use of multi-laser fluorescence-activated cell sorters. Cell sorters, traditionally, struggle to provide high cell viability due to harmful sorting pressures and applied charges. Additionally, their nondisposable fluidics can cause contamination from previous experiments.
The WOLF G2 addresses these issues with up to 9-color detection (3 configurations), low pressure (< 2 psi), and singleuse sterile cartridges and fluidics. Here we use our 488/561 nm configuration to dual-sort CD4+ and CD8+ T cells with 5 of the available channels.
Method
Veri-Cell PBMCs (BioLegend, #425004) were prepared according to the manufacturer instructions and diluted in HBSS/2% FBS (sample buffer). 1 x 106 cells were brought to 50 μL for staining in sample buffer and blocked with 10% FBS (GenClone, #25-514H) and 5% True-Stain Monocyte Blocker (BioLegend, #426102) for 10 minutes at room temperature. Cells were then stained with FITC CD8, PE-Cy5 CD3, PE CD4, PE-Dazzle 594 CD19, and PE-Cy7 CD45 (BioLegend, #301006, 300410, 300508, 562321, 304016) for 10 minutes at 4oC in the dark. They were then washed twice with sample buffer, resuspended at approximately 3 x 105 cells/mL, and passed through a 35 μm strainer (FlowTubes, #T9005) before analysis.
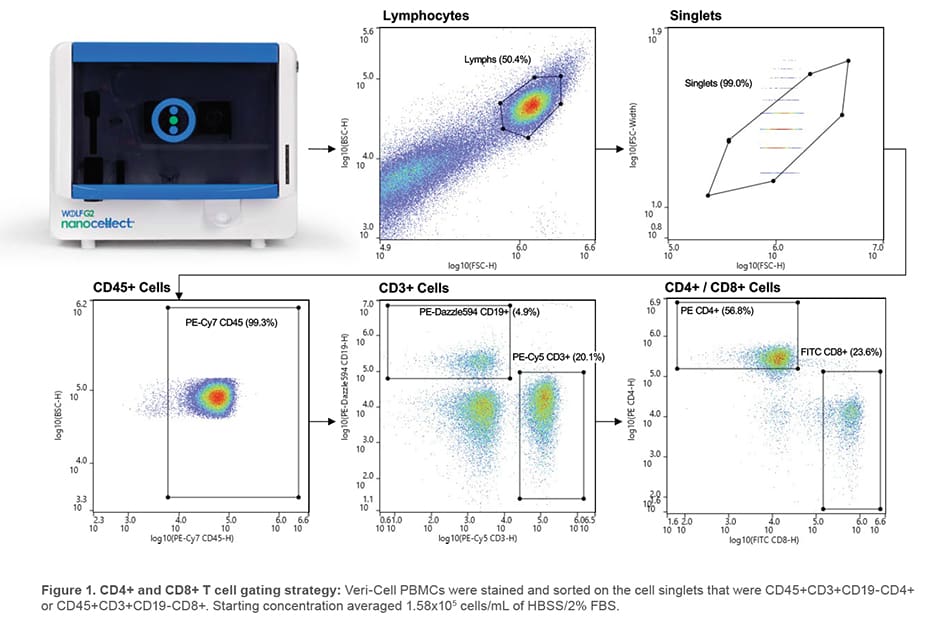
2 mL of sample was dual-sorted for lymphocyte singlets that were CD45+CD3+CD19-CD4+ or CD45+CD3+CD19-CD8+ (Figure 1). FITC CD8 was evaluated with the 488 nm laser and remaining antibodies were evaluated with the 561 nm laser. Sorted cells were centrifuged at 350 x g for 5 minutes, resuspended in 300 μL sample buffer, and split to analyze purity on the WOLF G2 and third-party analyzer.
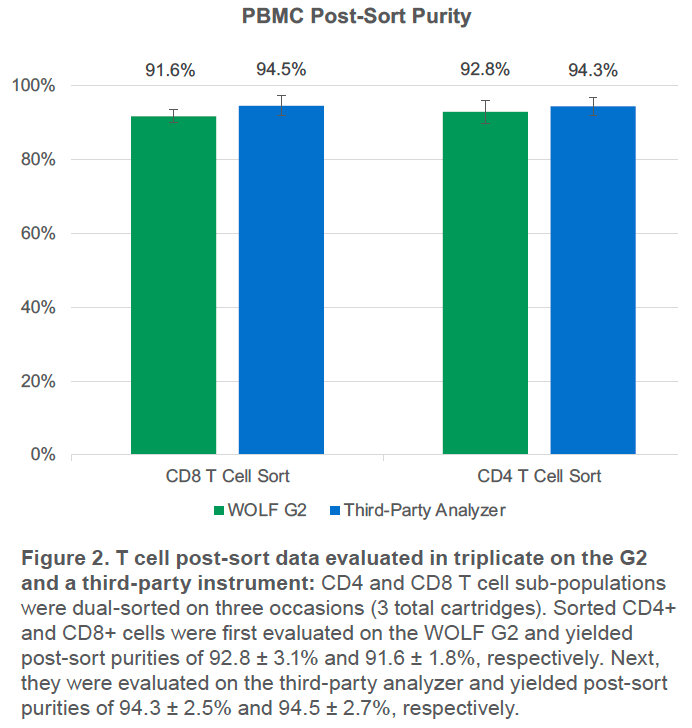
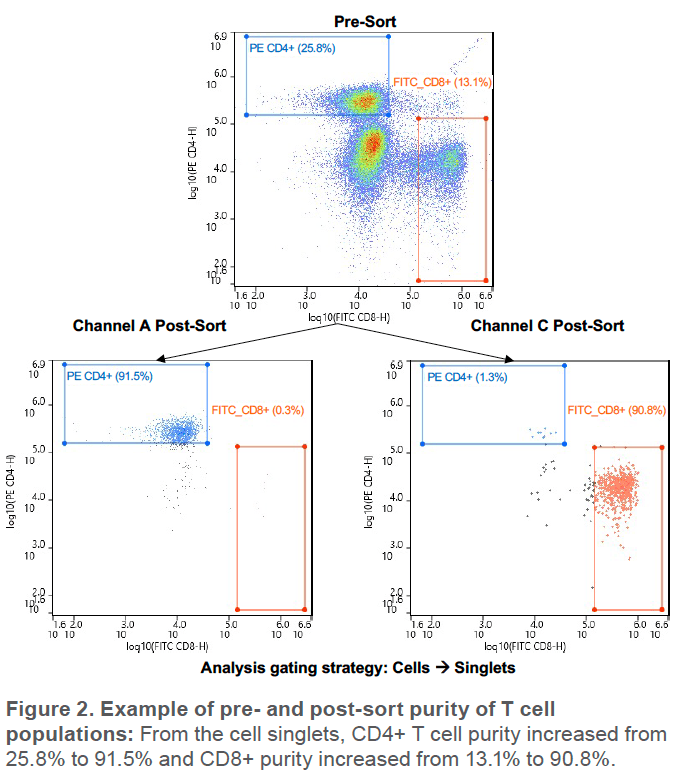
The above was repeated for a total of 3 cartridges. For each cartridge, an average of 6,500 CD8+ and 22,000 CD4+ events were sorted, starting from average purities of 14.3% and 27.9%, respectively. Lowest starting purity was 13.1% for CD8+ cells (Figure 2)
Results
The WOLF G2 was able to successfully purify both CD4+ and CD8+ T cell populations. Third-party analysis of sorted cells indicates purity over 94% for both populations and WOLF G2 analysis indicates purity over 91% for both populations (Figure 3). The small difference in purities is likely due to variation in gating, fluidics, and optics but both still indicate high purity, > 91%.
Conclusion
The G2 successfully sorted CD4+ and CD8+ T cell subsets with an average purity of > 91% with WOLF G2 analysis and > 94% with third-party analysis. The higher purity on the third party analyzer is a strong indication of success since it has very high resolution and low carryover. Though sorting more CD8+ events would further increase the strength of the results, the agreement across 3 replicates and similar sorting efficiency to the CD4+ cells indicate robust results.
Here we used only 5 of the available 9 detection channels. More sophisticated experiments are possible while taking advantage of the WOLF G2’s high viability, sterility, and purity.
For more information, visit nanocellect.com or email [email protected]
References
1. Sommermeyer, D., Hudecek, M., Kosasih, P. L., Gogishvili, T., Maloney, D. G., Turtle, C. J., & Riddell, S. R. (2016). Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia, 30(2), 492–500. https://doi.org/10.1038/leu.2015.247
2. Rha, M.-S., Jeong, H. W., Ko, J.-H., Choi, S. J., Seo, I.-H., Lee, J. S., Sa, M., Kim, A. R., Joo, E.-J., Ahn, J. Y., Kim, J. H., Song, K.-H., Kim, E. S., Oh, D. H., Ahn, M. Y., Choi, H. K., Jeon, J. H., Choi, J.-P., Kim, H. B., … Shin, E.-C. (2021). PD-1-Expressing SARS-CoV-2-Specific CD8+ T Cells Are Not Exhausted, but Functional in Patients with COVID-19. Immunity, 54(1), 44–52. https://doi.org/10.1016/j.immuni.2020.12.002
3. Provine, N. M., Larocca, R. A., Aid, M., Penaloza-MacMaster, P., Badamchi-Zadeh, A., Borducchi, E. N., Yates, K. B., Abbink, P., Kirilova, M., Ng’ang’a, D., Bramson, J., Haining, W. N., & Barouch, D. H. (2016). Immediate Dysfunction of Vaccine-Elicited CD8+ T Cells Primed in the Absence of CD4+ T Cells. The Journal of Immunology, 197(5), 1809–1822. https://doi.org/10.4049/jimmunol.1600591
APN -026