Monoclonal cell line generation from small-scale to high-throughput labs
Generating monoclonal cell lines is essential for a variety of scientific applications. From ensuring uniform gene expression in genetically engineered disease research lines to stabilizing production of antibodies for industrial manufacturers, there is a ubiquitous need for labs to isolate single cells for monoclonal cell line expansion. However, despite the demand for quick and cost-effective options, most labs still utilize the manual and inefficient cell cloning method known as limiting dilution (LD).
Manual limiting dilution methods limit productivity
According to Poisson distribution statistics,1 when cells are diluted and dispensed at 1 cell/well, 37% of wells should contain a single cell. More dilute concentrations can further eliminate doublets, with expected frequencies of 19% to 30% single-cell wells for 0.25 to 0.50 cells/well concentrations, respectively. These statistics are often inflated by the assumption that all viable clones have the same random chance of monoclonality,2 however, even under perfect conditions, 2/3 of each plate is still rendered useless. This wastes a large amount of supplies and time to produce sufficient monoclonal colony numbers.
Improve time, cost, and expression efficiency with the WOLF
The NanoCellect WOLF Cell Sorter significantly reduces all these resources wasted by the limiting dilution method. The WOLF and N1 single cell dispenser use sterile disposable microfluidic cartridges to analyze, sort, and dispense single cells into 96- or 384-well plates in as little as 8 minutes with over 90% efficiency.3 The WOLF is small enough to fit within a biosafety cabinet to maintain sterility; it provides 2 scatter and 3 fluorescent channels for selection of your cells of interest. Below, Chinese Hamster Ovarian (CHO) cells, the leading cell line used by industrial manufacturers for protein production, are used to demonstrate the substantially higher efficiency of the WOLF when compared with a range of limiting dilution concentrations.
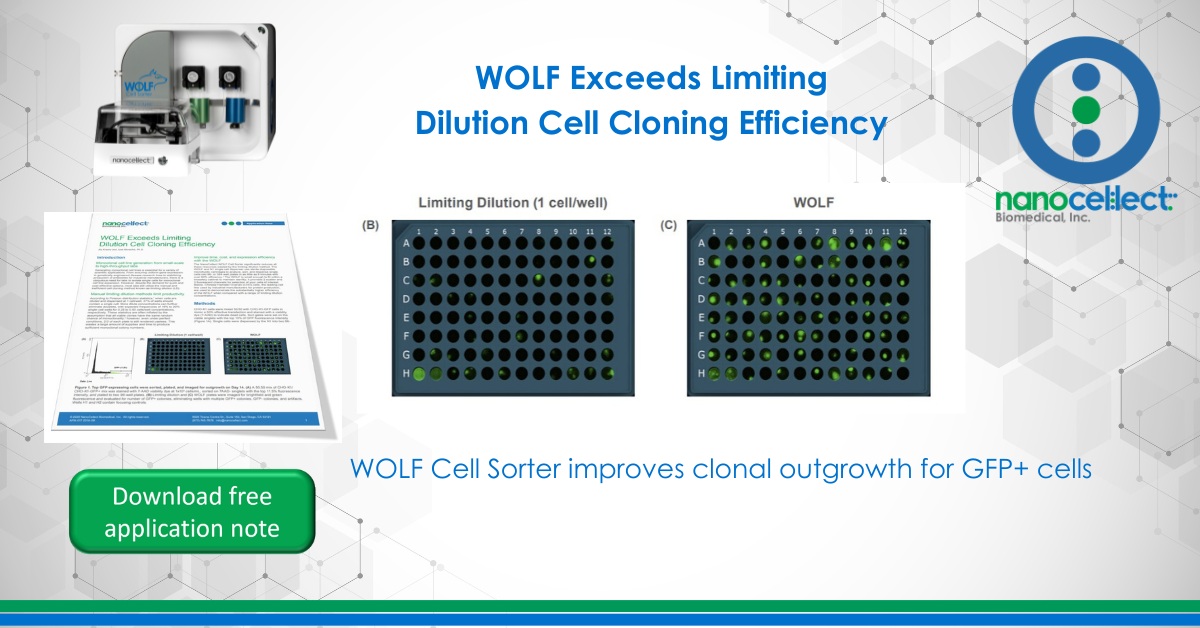
CHO-K1 cells were mixed 50:50 with CHO-K1-GFP cells to mimic a 50% effective transfection and stained with a viability dye (7-AAD) to indicate dead cells. Sort gates were set on the viable singlets with the top 10% of GFP fluorescence intensity (Figure 1A). Single cells were dispensed by the N1 into two 96-well tissue culture-treated plates filled with Ham’s F-12 media, supplemented with 10% FBS and 2x Antibiotic-Antimycotic. The 50:50 mix of CHO-K1 and CHO-K1-GFP cells was also diluted and plated at 0.25, 0.5, and 1 cell/well into two 96-well plates per condition and all plates were incubated for two weeks. Extra cells were added to wells H1 and H2 to facilitate focusing prior to imaging each plate. Brightfield and green fluorescence images were captured for each plate on Day 0 and Day 14 on the SYNENTEC’s NyOne™ (Figures 1B, 1C).
WOLF increases monoclonal colony count 8-fold and doubles expression level
After culturing the cells for 14 days, plate images were manually evaluated for clonal outgrowth. Compared to the limiting dilution method, there was a significant increase (up to 8-fold) in the abundance of GFP+ monoclonal colonies dispensed by the WOLF (Figure 2A). The colony quality was also improved, with maximum clone fluorescence intensity almost twice as high as the limiting dilution clones (Figure 2B).
Eliminate the need for downstream characterization
Dispensing with the WOLF decreased GFP- colony outgrowth 30-fold, yielding only a single colony across two plates (Figure 2A). With this low level of off-target interference, downstream clone characterization needs may be decreased or even eliminated.
Using the WOLF for single cell cloning assays results in a significant decrease in plating time, supplies needed, and increases the gene expression levels in the lead bioproduction cell line. In conclusion, the WOLF Cell Sorter can drastically increase efficiency and productivity of any cell cloning workflow.
APN-017





