Performance Verification of NanoCellect® Cartridges
Introduction
NanoCellect® has developed a third generation of microfluidic cartridges for use on the WOLF® and WOLF G2® Cell Sorters. The material for the new cartridges has improved injection moldability, low autofluorescence, and production methods enabling higher production volumes. Here, the performance of our previous WOLF Cartridge was compared to the new NanoCellect Cartridge using bulk and single-cell sorts to evaluate sorting efficiency and post-sort cell viability.
Methods
Bulk Sort Purity
NanoCellect Cartridges were used for bulk sorting to compare post-sort purity to historical data from the WOLF Cartridges.
Samples were prepared in 0.22 μm filtered 1X PBS (BioPioneer #MB1011-1X) buffer that was also used as sheath buffer. The sample was composed of three target populations: 10% 7 μm Dragon Green (DG) beads (Bangs Labs #FSDG007), 10% 15 μm DG beads (Bangs Labs #FCDG011), and 80% 10 μm Envy green beads (Bangs Labs #FSEG008).
Each stock was diluted to a range of concentrations: 1,200,000 beads/mL, 600,000 beads/mL, 300,000 beads/mL, and 100,000 beads/mL. The samples were evaluated pre-sort and post-sort using a third-party flow cytometer to measure and verify starting concentrations and purity after sorting. Each sample type was sorted multiple times on the WOLF G2. The 10% population of 7μm DG beads were sorted to channel A in the sorting junction, and the 15% 15 μm DG beads were sorted to channel C. Sorts were repeated on five NanoCellect Cartridges with seven repeats of one-way sorts to each channel A and channel C. Seven repeats of two-way sorts to both channel A and C were performed at a concentration of 100,000 cells/mL.
Bulk Sort Viability
NanoCellect Cartridges were used to measure equivalent post-sort viability with WOLF Cartridges. CHO-K1 cells were evaluated for viability after being sorted using the cartridges. The cells were unlabeled and sorted based off a cells gate followed by a singlets gate to eliminate debris and doublets. These bulk sorts were done for 25 minutes each.
CHO-K1 cells were sorted on both cartridge types at a starting concentration of 3.0 x 105 cells/mL in sorting buffer composed of 1X PBS, 0.5% BSA (Thermo Scientific #37525), 12.5 mM HEPES (Gibco #1560080), and 0.5 mM EDTA (Invitrogen #15575020).
Unsorted and sorted cells were stained with DAPI (Invitrogen #R37606) and evaluated for viability on a third-party flow cytometer. In addition, a dead control was prepared by heat- killing CHO-K1 prior to staining. The sorts were repeated on three NanoCellect Cartridges and three WOLF Cartridges.
Single-Cell Dispensing Efficiency with Beads
Single cell sorting performance was evaluated on both NanoCellect and WOLF Cartridges. 15 µm DG beads were diluted in PBS to 1.0 x 105 beads/mL and sorted into 96-well and 384-well plates with the WOLF G2 and N1 Single-Cell Dispenser. The plates were imaged with the NyOne® (SYNENTEC) to determine the number of wells containing single beads. A total of 7 cartridges were used for verification of single-cell efficiency.
Single-Cell Dispensing Outgrowth
Long-term outgrowth was compared between the NanoCellect Cartridges and WOLF Cartridges. CHO-GFP cells were diluted to 1.0 x 105 cells/mL in sorting buffer composed of 1X PBS, 0.5% BSA, 12.5 mM HEPES, and 0.5 mM EDTA. For each cartridge, three 96-well plates pre-filled with Ham’s F-12K (Kaighn’s) Medium (Gibco #21127022), 10% FBS (Gibco #10082147), and 1X antibiotic-antimycotic (Gibco #15240062). CHO-GFP were sorted off a scatter plot parental gate, followed by a singlets gate, and finally a fluorescent gate to sort out the top 30% GFP- expressors. After sorting the cells, the plates were incubated at 37°C, 5% CO2 for 7 days. Cells were imaged on the Celigo (Revvity) to detect number of wells containing a single cell on day 0 and to later confirm single clone outgrowth on day 4.
Results
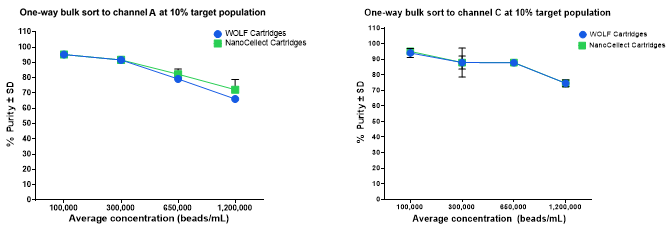
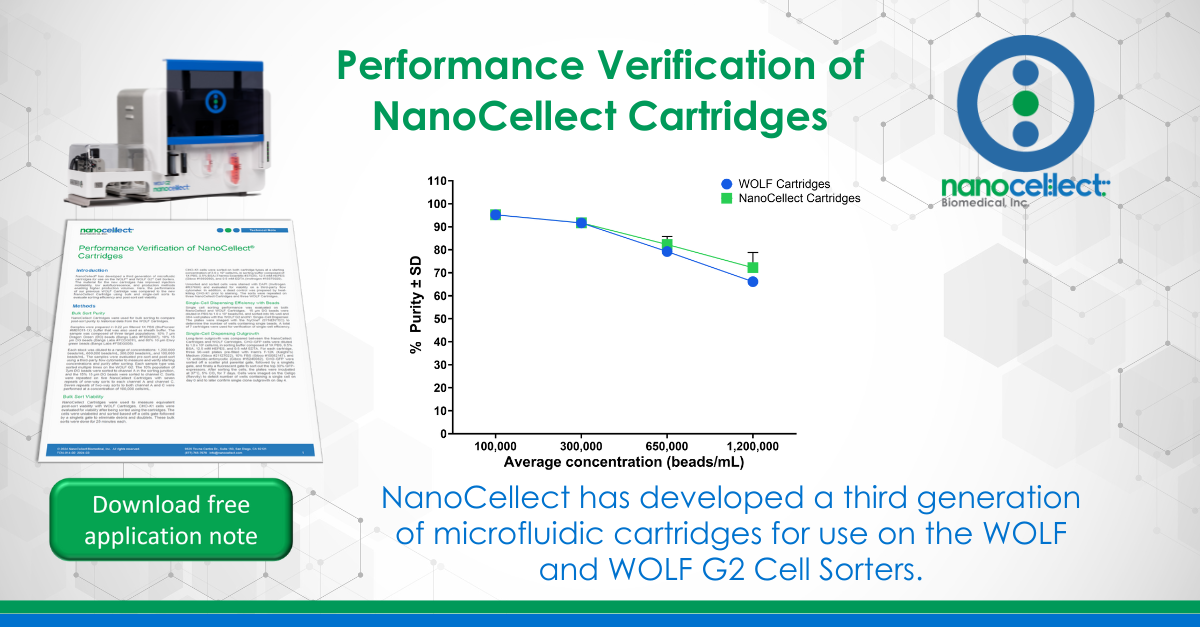
Figure 1. Post-sort purity is similar for both cartridge types across multiple target populations and concentrations when sorting a single target population. (A) The 10% 7 μm Dragon Green (DG) bead target population was sorted at 4 total bead concentrations using a one-way sort towards channel A; each concentration was sorted multiple times per cartridge. n = 8, 6, 3, and 3 sorts for 1,200,000, 650,000, 300,000, and 100,000 beads/mL, respectively. (B) The 10% 15 μm DG bead target population was sorted at 4 total bead concentrations towards channel C; each concentration was sorted multiple times per cartridge. n = 8, 6, 3, and 3 sorts for 1,200,000, 650,000, 300,000, and 100,000 beads/mL, respectively. For both data sets, historical data from WOLF Cartridges was tested under the same criteria and displayed for comparison.
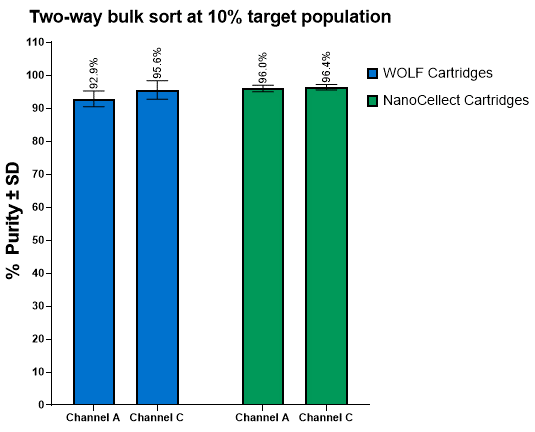
Figure 2. Post-sort purity is similar for both cartridge types during a two-way sort at 100,000 cells/mL. The 10% 7 μm DG bead target population was sorted to channel A and a 10% 15 μm DG bead target population was sorted to channel C. The two-way sort showed comparable results to the historical data from WOLF Cartridges. A total of two cartridges of each kind was tested with seven two-way sorts performed.
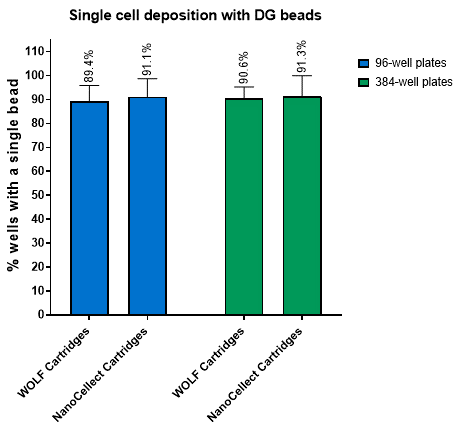
Figure 3. Single-cell dispense efficiency is similar for both cartridge types across multiple plate types. DG beads were sorted based on FL1- fluorescence into 96-well or 384-well plates (n = 7 cartridges). Plates were imaged on the NyOne and counted for wells containing single beads out of total wells to determine the average dispense efficiency. Both cartridge types show comparable results.
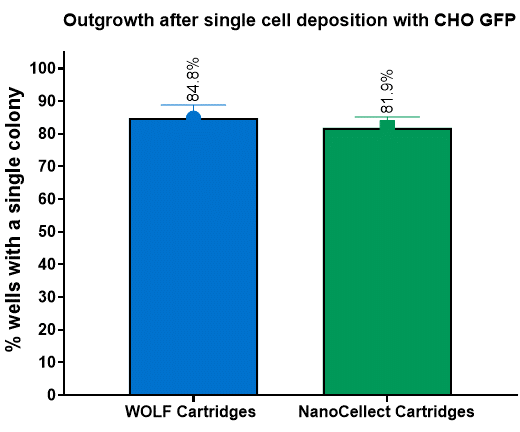
Figure 4. Post-sort outgrowth is similar for both cartridge types across: CHO-K1-GFP cells were sorted based on GFP expression fluorescence by each cartridge into three 96-well plates pre-filled with optimal cloning media. This was repeated on three cartridges for each type tested. The plates were imaged on day 0 to determine which wells have a single cell and analyzed through day 7 to determine its outgrowth. Both cartridge types do not show differences in outgrowth.
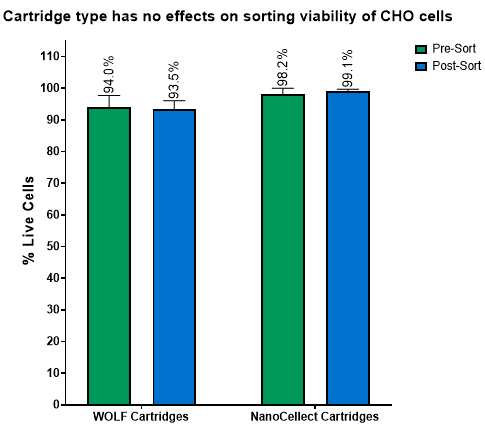
Figure 5. Post-sort viability of CHO-K1. CHO-K1 cells were sorted from a scatter plot. The sort was performed for 25 minutes followed by staining for dead cells. A total of 3 cartridges was used for this test. They are no differences in viability between cartridge type. In addition, that the cartridge did not induce additional cell death.
NanoCellect Cartridges were evaluated with respect to their bulk sorting performance, and compared to historical data sets obtained from WOLF Cartridges. One way bulk sorting of 15 μm DG beads was performed at various input sample concentrations and the resulting post-sort purity was measured (Figure 1). However, sorting demonstrated that increasing the input sample concentration yields a decrease in post-sort purity.
NanoCellect Cartridges possessed equivalent or enhanced post-sort purity outcomes compared to WOLF Cartridges, even when using a two-way sort when sorting 15 μm DG beads (Figure 2). Furthermore, the NanoCellect Cartridges were also used to measure single cell deposition between beads and cells with results showing similar efficiencies compared to WOLF Cartridges (Figure 3). This was further demonstrated with cells growing up to day 7 and showing similar outgrowth rates (Figure 4). Lastly, when sorting CHO cells via bulk sort to demonstrate viability, both cartridges performed similarly with the cartridges not affecting viability (Figure 5).
Conclusion
NanoCellect Cartridges demonstrate similar performance to WOLF Cartridges and maintain important key features such as low-pressure cell sorting, optical clarity, and sterility. The results were similar when sorting using bulk or single cell for purity and viability. Therefore, NanoCellect Cartridges offer fluidic improvements with equivalent performance.
For more information, visit nanocellect.com or email [email protected].
TCN-014
