When trying to isolate a rare target population, researchers may decide to perform an enrichment beforehand to shorten sorting time and increase purity. For example, regulatory T cells (Tregs) make up 1-4% of the peripheral blood mononuclear cells (PBMCs) and 5-10% of the CD4+ T cell population. In order to reduce the sorting time, a CD4-based enrichment can be performed prior to increase the proportion of Treg target cells. Pre-enrichment is a common practice that can be performed with magnetic cell isolation technology or fluorescent cell sorters. However, traditional cell sorters that use droplet-based sorting create high shear stress on cells that can result in a less viable sample post-enrichment. Here, we demonstrate that gentle microfluidic cell sorting can be performed with high sample concentrations to pre-enrich samples.
PBMCs (SD Blood Bank) were isolated using Lymphopure (BioLegend #426201) and stained with CD4-PE (BioLegend #317410). PBS + 1% BSA was used as the sample and sheath buffer. Each donor’s sample was prepared at various concentrations: 4-6 x 105 cells/mL, 1-2 x 106 cells/mL, 3-4 x 106 cells/mL and 5 x 106 cells/mL. Samples at each concentration were analyzed with FSC, BSC and CD4-PE fluorescence. CD4+ cells were sorted on the WOLF® Cell Sorter for 30 minutes processing 720 μL of sample. The cartridge was examined after each sort to determine if any clogging had occurred. Each concentration was repeated 3-4 times. Post-sort purity was assessed on the Novocyte (Agilent).
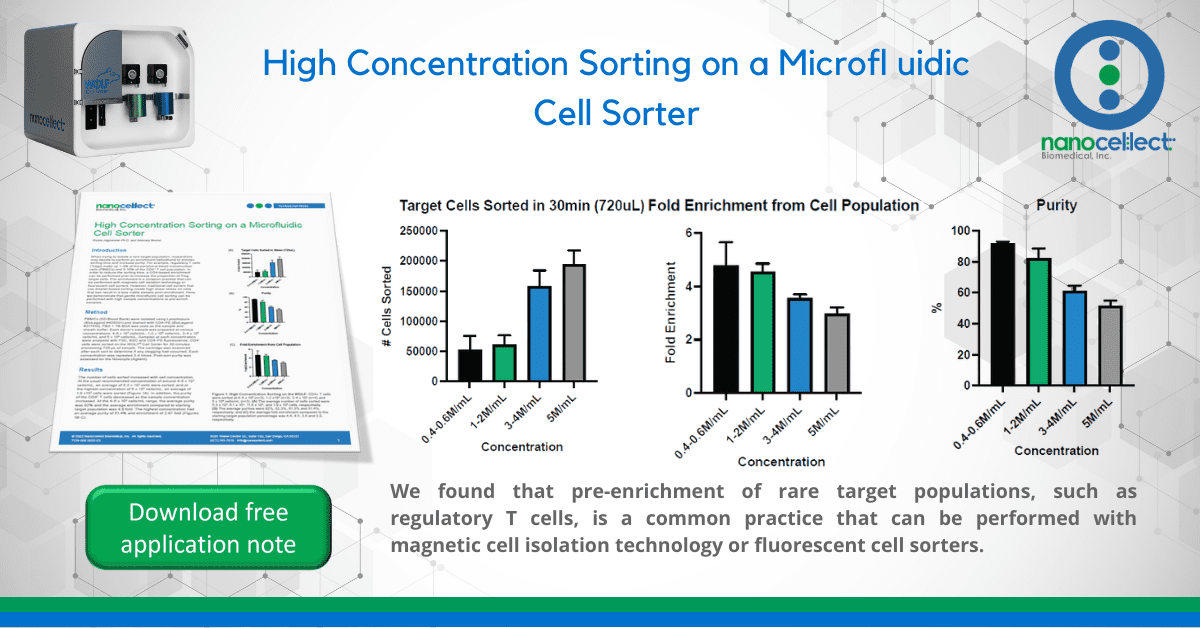
The number of cells sorted increased with cell concentration. At the usual recommended concentration of around 4-6 x 105 cells/mL, an average of 5.3 x 104 cells were sorted; and at the highest concentration of 5 x 106 cells/mL, an average of 1.9 x105 cells were sorted (Figure 1A). In addition, the purity of the CD4+ T cells decreased as the sample concentration increased. At the 4-6 x 105 cells/mL range, the average purity was 92% and the average enrichment compared to starting target population was 4.8 fold. The highest concentration had an average purity of 51.4% and enrichment of 2.97 fold (Figures 1B-C).
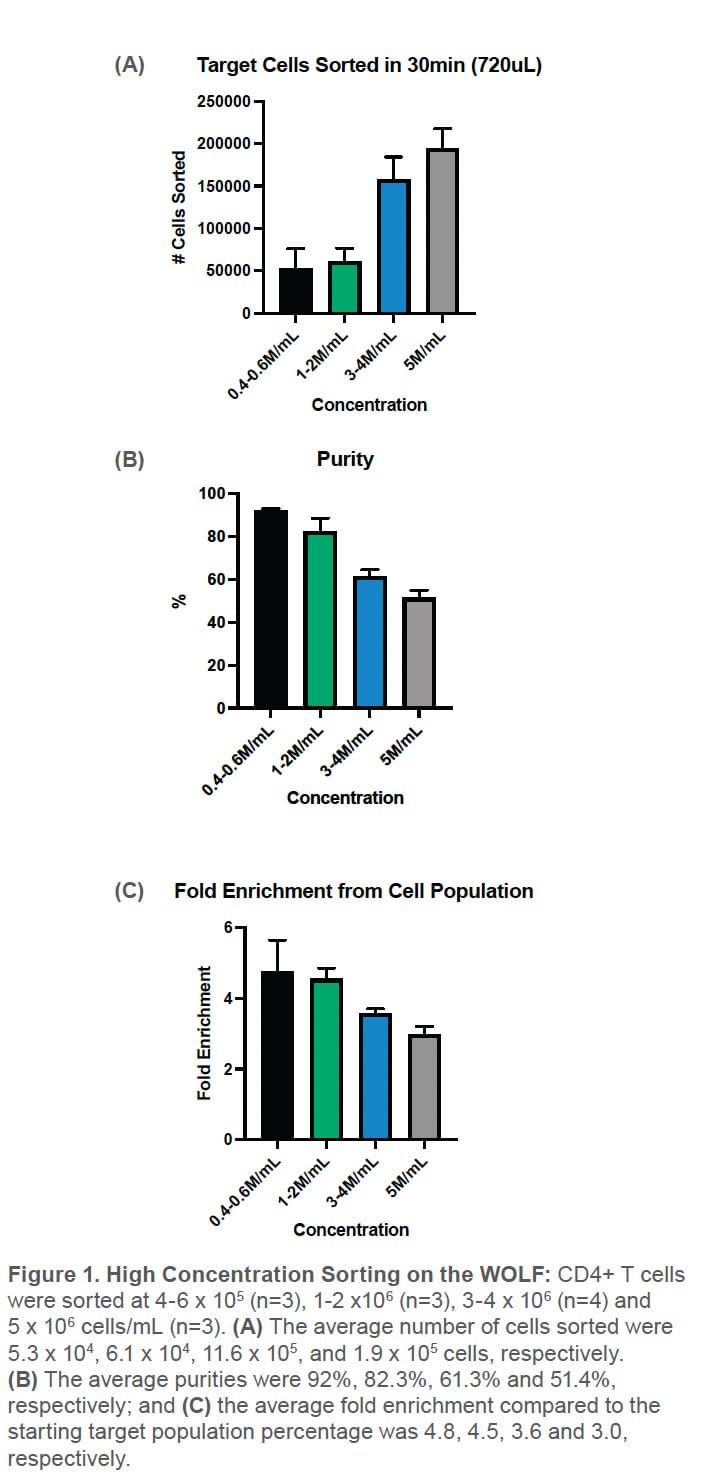
This experiment shows that NanoCellect’s microfluidic sorting cartridges can be used at higher concentrations; however, this results in lower purity consistent with concentration-dependent Poisson effects. Experiments with low-percentage target cell populations may benefit from this two-step sorting method by first performing an enrichment sort to increase the target population and reduce sorting time. No clogging was observed with the PBMCs using PBS +1% BSA as the sheath and sample buffer. Concentrations above the 5 x 106 cells/mL are at high risk of clogging and are therefore not recommended for the microfluidic cartridges. In addition, clogging may also occur at lower concentrations with other sample types. Always test out lower concentrations first and gradually increase if no clogging is observed. In conclusion, these experiments demonstrate that higher concentrations can be run with NanoCellect’s microfluidic cartridges for users who want to perform a two step enrichment protocol for rare cells.
For more information, visit nanocellect.com or email [email protected]
TCN – 008





