7-Color Panel Design on the WOLF G2® Cell Sorter with 488nm/637nm Laser Configuration
Introduction
Multiparameter flow cytometry is a powerful tool that enables scientists to identify numerous cell types simultaneously with the use of fluorescent antibodies, and then correlate their internal cellular processes. This is of utmost importance for scientists wanting to visualize an immune response or cell-cell interactions after infection or vaccination, for example. The WOLF G2® Cell Sorter is a microfluidics-based platform that uses sterile, disposable cartridges to analyze and sort cell populations with low sample pressures (< 2 psi). The sorter is offered in four different configurations: 488nm single laser, 405nm/488nm, 488nm/561nm, and 488nm/637nm two-laser systems. Herein, we demonstrate the separation of seven cell types using a 7-color PBMC panel, in addition to the enrichment of CD8+ and CD4+ T-cells on the WOLF G2 with 488nm/637nm configuration.
Methods
A 7-color antibody panel was designed using BioLegend’s FluoroFinder panel builder (https://www.biolegend.com/en-us/panel-builder), multicolor panel selector (https://www.biolegend.com/en-us/panel-selector), and WOLF Spectra Viewer (https://nanocellect.com/wolf-spectra-viewer/).
As per manufacturer’s instructions, Veri-Cells™ PBMCs (BioLegend cat# 425004) were reconstituted in Buffer A, aliquoted into sample tubes, and then blocked with Human TruStain FcX™ (BioLegend, cat# 422301) for 10 minutes to minimize non-specific binding of the antibodies. The cell samples were then stained with anti-human BioLegend antibodies (Table 1): CD14 AlexaFluor 488™ (cat# 301817), CD4 PE (cat# 300507), CD19 PE-Dazzle 594™ (cat# 302251), CD56 PE-Cy7 (cat# 318317), CD45 PerCP (cat# 304025) CD8a AlexaFluor 647™ (cat# 300918), and CD3 APC-Cy7 (cat# 300317).
Antibody titrations were conducted to ensure good separation of negative from positive signal and to prevent oversaturation of antibodies. Compensation beads (Slingshot Biosciences, cat# SSB-05-B) were used to apply compensation to the samples. Gate strategy and placement was confirmed through back-gating and fluorescence minus one (FMO) controls.
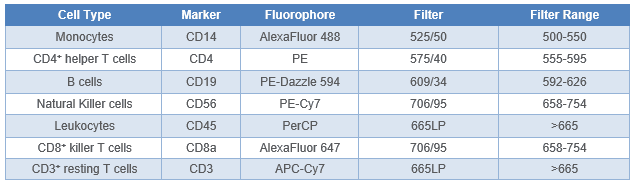
Table 1. 7-Color VeriCell™ PBMC panel design. Filter setup on the WOLF G2 with 488 nm/637 nm laser configuration are stated.
Results
All seven antibodies and their corresponding cell types were successfully identified on the WOLF G2 with the 488nm/637nm configuration. The gating strategy was as follows: PMBCs were identified and separated from background noise and debris through a BSC-H vs. FSC-H scatter plot (Figure 1A). Singlets were then identified using a FSC-Width vs. FSC-H scatter plot (Figure 1B). From the singlets gate, leukocytes were identified through a BSC-H vs. PerCP CD45-H scatter plot (Figure 1C). From the CD45+ parent gate, CD19+ B-cells were identified using a BSC-H vs. PE Dazzle-594-CD19-H scatter plot (Figure 1D). CD3+ lymphocytes were also identified from the CD45+ parent gate on a BSC-H vs. APC Cy7-CD3-H scatter plot (Figure 1E). CD8+ T-cells and CD4+ T-cells were then identified using the CD3+ child gate on a scatter plot of AlexaFluor 647-CD8-H vs. PE-CD4-H (Figure 1F). Natural killer cells were identified from the parent CD45+ gate on a scatter plot of BSC-H vs. AlexaFluor 488-H (Figure 1G). Lastly, monocytes were identified independently using a scatter plot of BSC-H vs. AlexaFluor 488-DC14-H gated to CD45+ parent (Figure 1H).
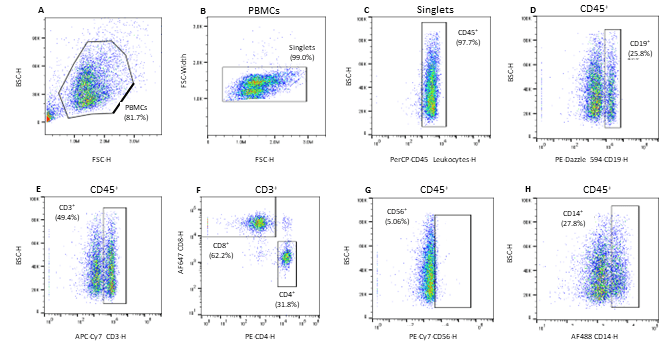
Figure 1. Gating strategy for the identification of 7 cell subpopulations in Veri-Cell™ PBMCs: A. PBMCs were gated using the BSC-H/FSC-H scatter plot, B. PBMC singlets were then identified via FSC-W/FSC-H scatter plot gated to the PBMC gate, C. CD45+ leukocytes were identified on a BSC-H/PerCP CD45-H scatter plot gated to singlets, D. CD19+ B cells were identified using the CD45+ parent gate on a BSC-H/PE Dazzle 594-H scatter plot, E. CD3+ lymphocytes were identified using a BSC-H/APC-Cy7 CD3-H scatter plot gated to the parent CD45+ population, F. CD3+ child gate was used to identify the CD8+ killer T-cells and CD4+ helper T-cells on a AF647-CD8-H/PE-CD4-H scatter plot, G. CD56+ Natural Killer (NK) cells were identified on a scatter plot of BSC-H/PE-Cy756-H gated to CD45+ parent gate, and H. CD14+ monocytes were identified from the CD45+ parent gate and further separated on a BSC-H/AF488 CD14-H scatter plot.
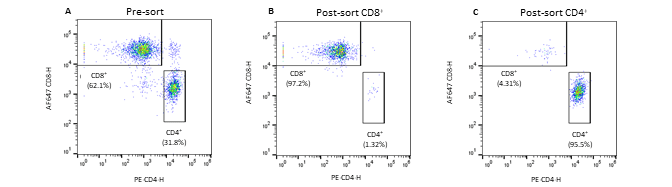
Figure 2. Pre- and post-sort enrichment of CD8+ and CD4+ T-cell populations on the WOLF G2 with a 488nm/637nm laser configuration: A. Pre-sort CD8+ and CD4+ cell populations, B. Post sort enrichment for CD8+ T-cells, C. Post sort enrichment for CD4+ T-cells. All scatter plots are initially gated from the CD3+ cell population. CD8+ and CD4+ cell populations were enriched from 55.4% and 37.3%, respectively, to 97.1% and 96.5% respectively.
Conclusion
Seven fluorescent antibodies were successfully used to identify seven cell types within a PBMC population. Furthermore, the WOLF G2 with a 488nm/637nm laser configuration enriched for CD8+ and CD4+ T-cells independently to approximately 97% purity. The data demonstrates the broad capabilities of the WOLF G2 in multiparameter flow cytometry.
For more information, visit nanocellect.com and abterrabio.com or email info@nanocellect.com
TCN-011
