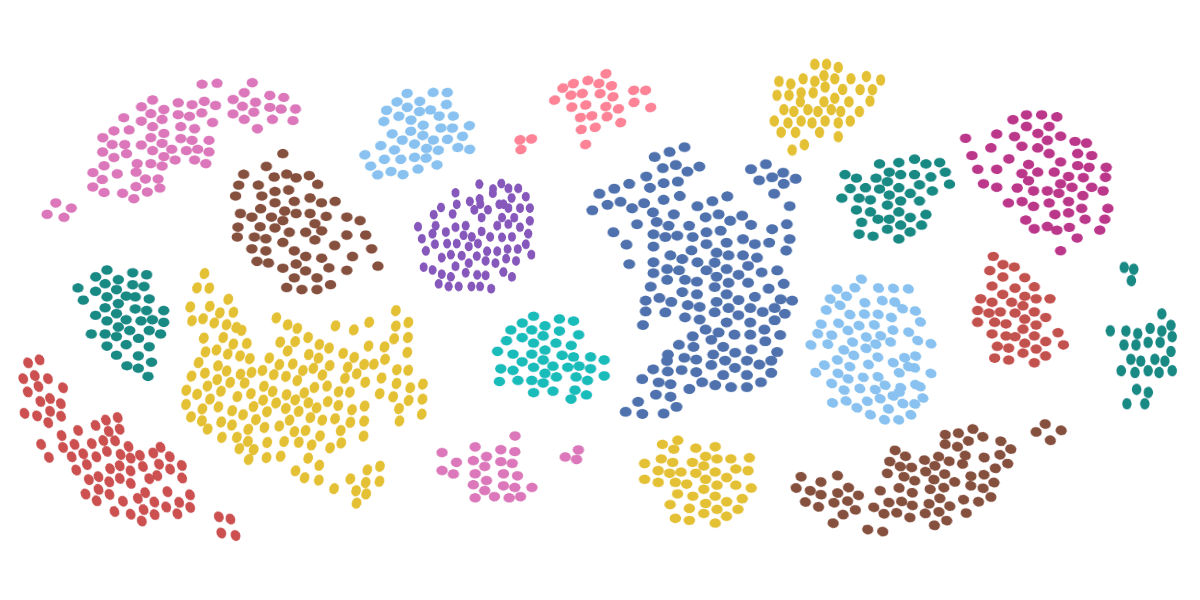
Breaking Down the Principles of Flow Cytometry
Flow cytometry is a useful technique used in cell biology, cancer testing and research, stem cell research, and many other fields. Using a flow cytometer is a method of measuring—organizing, in as sense—microscopic cells found in a heterogeneous solution.
If you’re reading up on this topic as a refresher, welcome. If you’re new to flow cytometry, don’t worry; this guide is here to break down flow cytometry and the basic principles behind its utility.
Flow Cytometry | A First-Timer’s Guide
Already know the basics of flow cytometry? Skip ahead to Applications of Flow Cytometry.
If you’re new, put on your goggles, strap on your fluorescence gear, and prepare to get excited. If you didn’t laugh at this pun, you will soon.
What Is Flow Cytometry?
In its essence, “Flow” refers to fluid or motion. “Cyto-” is a prefix, often used in biology that relates to “cell” or “cells” and “-Metry” relates to “measure” or the “process of measuring.”
Thus, putting it all together:
- Flow cytometry is the process of measuring cell properties in a fluid stream.
Principles of Flow Cytometry
Flow cytometry uses three basic scientific principles—fluid dynamics, optics, and electronics—to detect, count, and do cell sorting. This process solves two primary pain points:
- Consider that prokaryotic cells have diameters as small as 0.1 µm (micrometers, or 0.1 x 10-6 meters), and eukaryotic cells have diameters up to 100 µm. Measuring, analyzing, and sorting cells of this size manually is either impossible or incredibly time consuming.
a) Thus, electronic systems are needed to calculate and sort in real time. And the electronic systems can be informed using optic systems. - When collecting samples large enough to run experiments and tests on, you’ll rarely find any homogeneous fluid sample.
a) Think about blood or saliva, two common fluids that are used to run diagnostics, test medications, or identify phenotypes. Blood contains five types of white blood cells, red blood cells, and platelets. Saliva has proteins, enzymes, hormones, genetic material, and a variety of other biomarkers.
Because of these, flow cytometry is incredibly useful for measuring and analyzing the cells one at a time. How does it accomplish this?
Flow Cytometry Principle 1 | Fluid Dynamics
The first step in flow cytometry is taking a flow cytometry test – taking a heterogeneous sample and injecting it into the sheath fluid.
- Sheath Fluid – Commonly a buffered saline solution, sheath fluid runs through a flow cytometer at laminar flow.
- Laminar Flow – Movement where each layer of liquid moves past adjacent layers with very little to no mixing.
Because of hydrodynamic focusing, when the heterogeneous sample is injected into the sheath fluid (at a slightly higher pressure), the cells migrate into a single-file line to be passed through a laser beam.
Aha! So this is where optics comes in, huh? Right you are.
Flow Cytometry Principle 2 | Optics
With flow cytometers, thousands of cells can be analyzed and sorted per second. But what allows for these precise measurements at such a high speed? The first part comes from laser optics.
When the cells stream past the laser one at a time, their cell structure refracts part of the beam. Some of the light refracts past the cell to a detector behind, while other detectors sit at a 90-degree angle to measure the side refracted light.
- Forward Scatter (FSC) – Light scattering past the cell typically identifies the relative cell size. The intensity measured on the light scatter detector correlates with diameter.
-
- How is this principle of flow cytometry useful? Differentiating between white blood cells is one area where forward scatter plays a role.
-
-
- Lymphocytes: average diameter = 8.5 µm
- Neutrophils: average diameter = 8.85 µm
- Monocytes: average diameter = 15 µm
- Eosinophils: average diameter = 14.5 µm
- Basophils: average diameter = 15 µm
-
Clearly this won’t identify which of the five white blood cells are currently running through the laser. However, it does help differentiate them partly. To identify fully, FSC results must be combined with SSC.
- Side Scatter (SSC) – Light that is scattered perpendicular (90 degrees) is filtered through a number of lenses and mirrors and eventually runs through photomultiplying tubes (PMTs) where they’re collected into data points. Where FSC measures diameter and volume of the cells, SSC helps to provide information on the complexity of the cell. SSC can be used to determine the nucleus structure and granularity of the cell.
Combining these two detection methods allows for a great deal of differentiation between cells.
A question that has yet to be answered is how the laser “targets” each cell one at a time, despite being within a sheath fluid.
Fluorescently Labeled Cells | Time To Get Excited
Remember the hilarious pun you read earlier? Okay… the hilarious pun that you didn’t laugh at? Well, this has to do with how the heterogeneous fluid is “labeled.”
When oncologists want to measure and detect cancer cells in a patient’s body, a typical practice is for the patient to ingest radioactive iodine or another form of a radionuclide. The tissues surrounding the cancer (and some of the cancer cells themselves) will absorb the radioactive material and light up on the appropriate scan (PET scan, bone scan, etc.).
- If the cancer cells do not absorb the radioactive material, a “cold spot” can be detected in the scan.
- If the cancer cells do absorb the radioactive material, they’ll do so at a different rate than the surrounding tissue and create a “hot spot.”
Either way, this allows oncologists to point directly to cancer and measure its severity in a patient.
How does this relate back to flow cytometry? Great question.
Flow cytometry uses a similar principle. By utilizing a proper fluorescent label, otherwise known as a fluorochrome or fluorophore, you can target certain cells within a heterogeneous solution, or even target specific proteins within a cell itself. This can be achieved with fluorescently dyed and labeled antibodies that are highly specific that attach to normal cells or proteins. When the laser light hits the desired cell, the fluorescently dyed antibody gets excited and emits photons (causing the FSC and SSC to be detected).
Flow Cytometry Principle 3 | Electronics
To quickly recap, the cells to be measured are injected into a shear fluid at laminar flow. This creates the single-file march of cells toward the laser. The laser excites the fluorescently dyed cells, which create both forward scatter (FSC) and side scatter (SSC). Scattered light funnels through filters and mirrors to reflect into photo-multiplying tubes, detectors that are calibrated to detect a certain wavelength.
From here, the third and final principle applies: electronics.
Each PMT detector converts the fluorescence signal into a digital, trackable data point. These data points then create impressionistic images that can be analyzed to measure the different cells apparent in the heterogeneous fluid.
Applications of Flow Cytometry
The ability to measure multiple cell characteristics at one time with an automated process is invaluable to many different fields. And the most common applications include:
- Cell population sorting and cell isolation – When you want to create multiple homogenous solutions from one heterogeneous fluid, flow cell cytometry helps to identify and isolate cells. After the cells pass through the laser and the electronics systems records whether the single cell was fluorescently marked or not, the flow cytometer can place a charge (positive or negative) to the cells. Magnets then pulled the stream into three different directions (positive, negative, and neutral) and the cells are then isolated. Note that flow cytometry is a commonly used method to streamline the CRISPR process. If you’re interested in learning more about what is CRISPR, we’ve written in detail about this topic on our blog.
- Immunophenotyping – When you have a heterogeneous fluid and want to both identify and quantify each cell types within it, this is immunophenotyping. This process involves fluorescently labeling cells and cell proteins and is useful in clinical labs when diagnosing certain diseases. The cells taken are often looking for immune system biomarkers.
- Analyzing cell cycle – To analyze the four different stages of the cell life cycle, researchers can use different DNA-binding dyes. These cells can then be isolated and analyzed.
- Cell proliferation assays – The cells that are marked with fluorescent dye can be isolated and activated to undergo cell reproduction (mitosis). When the individual cell does multiply, the dye halves (roughly) between the two cells. This provides a direct way to measure proliferation of different cells.
Avoiding Cell Death | Benefits of the WOLF Cell Sorter
One of the largest pain points when operating flow cytometers is keeping the cells alive throughout the process. Because of apoptosis, cells that die within the cell sorter are not only useless for the secondary measurements and tests, but they create noise within the data.
Cells die from being over-oxygenated, under significant pressure, and of course, they die randomly as well.
In order to limit this pain, NanoCellect created the WOLF Cell Sorter. With a sorting pressure that is 30 times gentler than standard sorters (less than 2 psi), the WOLF Cell Sorter is able to help biopharmaceutical companies, labs, and more keep their cells alive when isolating.
Flow Cytometry Principles and Applications
In sum, to sort and analyze cells, it has to be done one cell at a time. Doing this manually is out of the question, and flow cytometers rely on basic principles to automate this process. Fluid dynamics and hydrodynamic focusing allows cells to stream past a laser one at a time. The principles behind optics allow the scattered light to be detected. And finally, electronic systems record the flow cytometry data to be analyzed further.
For a cell sorter that utilizes these principles and optimizes their effects to keep cells happy and healthy, think WOLF Cell Sorter.
Think NanoCellect.
Sources:
- NCBI. Hydrodynamic focusing – a versatile tool. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3251643/
- The Histology Guide. White blood cells. https://www.histology.leeds.ac.uk/blood/blood_wbc.ph
- American Cancer Society. Nuclear Medicine Scans for Cancer. https://www.cancer.org/treatment/understanding-your-diagnosis/tests/nuclear-medicine-scans-for-cancer.html
- Biocompare. Flow Cytometry Streamlines CRISPR Process. https://www.biocompare.com/Editorial-Articles/346273-The-CRISPR-Caveat/